Octopus (32 page)
Authors: Roland C. Anderson

A more natural but much more complicated way to remove nitrates is to use an algae turf scrubber. Some advanced hobbyists choose to use the scrubber to harvest and remove algae at regular intervals in order to help remove nitrates and other excess nutrients. No matter how nitrates are removed, all closed systems need time to establish colonies of bacteria. While cycling the system, you should not use chemical filtration (carbon, resins, protein skimmers, or even water changes) to compete with the fledgling bacteria colonies for nitrogen. To start cycling a marine system, you need a source of ammonia. The easiest and modern ethical method is to use chemical sources of ammoniaâammonium hydroxide or ammonium chloride. You will need a marine water testing kit to measure the levels of ammonia, nitrite, and nitrate, which can be purchased at any marine pet store.
You can buy ammonium hydroxide at grocery or hardware stores; get the pure stuff, free of other chemicals such as perfumes and cleaning substances that you don't want to introduce to your aquarium. Check the ingredients list and add some to a small bottle of water and shake; pure ammonia will not form foam. Since the amount of ammonia dissolved in water varies in ammonium hydroxide, most authorities simply suggest adding it until 5 ppm is reached. If you use a new bottle (the ammonia will evaporate if the bottle is left open) of ammonium hydroxide (28 percent ammonia) validated by the American Chemical Society (ACS), you can use the following approximate recipe as a guideline: one drop per gallon of water should get the desired 5 ppm concentration. Alternately, you can buy ammonium chloride (NH
4
Cl) in solid (salt) form from scientific laboratory supply stores. Unlike ammonium hydroxide, it has standard purity, and the recipe is more precise: add 0.017 oz. (0.055 g) of ammonium chloride per gallon of water to get the 5 ppm ammonia content.
There are many methods to cycle a tank. One method we like is to maintain a concentration of 5 ppm for the first ten days, 1 ppm for the next ten days, and stop adding ammonia altogether during the third ten-day period. During the end of the first ten-day period, you should see the nitrite levels rise, and during the second and third periods the nitrate levels will rise. A few days before adding the first hardy animals to the system,
say on day twenty-eight, do a 50-percent water change, and then attach the chemical filtration system, such as the protein skimmer and carbon or resins if you use them.
There are a number of “magic potions” that claim to help inoculate marine aquariums with bacteria. The products often claim to drastically reduce startup time. Some of these products help but many don't work at all. Even for those that can work, leaving them on the shelf of the aquarium supply store too long may mean that the bacteria are dead. The Nitrosomonas and Nitrobacter bacteria needed are aerobic, they require oxygen to survive, and they have a limited shelf life if sealed in a bottle. Since there is little quality control in these products and no shelf life printed on the bottle, don't rely on them. You can inoculate the system yourself by seeding it with gravel or the filter wash from a mature system. This approach provides a higher level of starting bacteria in strains that have already proven themselves successful in aquarium conditions. Whatever method you use, there simply is no substitute for patience when setting up closed marine systems.
During the cycling period, only biological filtration should be used. In other words, don't use chemical filters that will remove the ammonia and organics that will break down into ammonia. During this period, you actually want ammonia to develop to high toxic levels, so that you develop a strong population of bacteria to break it down.
After a month, hardy fish can be added to the system. The longer you wait with a biological load such as fish in the aquarium, the more stable it will become. Ideally, it is best to wait three months before removing the fish and adding an octopus. Octopuses eat a lot, grow fast, and produce more waste than a fish of a similar size, so take care not to add more and more biological load to your system until it crashes. Instead, remove the fish, add the octopus, and start using additional filtration while continuing to make regular partial water changes.
In addition to this brief introduction to marine systems, there are many aquarium stores, aquarium societies, books, magazines, and Web sites (such as
www.reefs.org
or
www.reefcentral.com
) dedicated to the subject of setting up and maintaining marine aquariums. We encourage readers interested in marine systems to learn more from these sources.
Keeping Your Octopus Healthy
Many people have expressed confusion about the requirements for keeping healthy octopuses. (In the past, there was limited guidance, but over the past decade, more accurate and helpful information has become available. Internet chat groups like
TONMO.com
and cephgroup are very useful.) Some of the confusion may be related to mysterious deaths that are simply the natural deaths of short-lived animals. Other deaths may be the result of elevated levels of trace elements that are deadly to cephalopods. Many sources state that octopuses demand optimal water quality and that they cannot endure any water pollution. But Roger Hanlon and John Forsythe (1985) have shown that octopuses are surprisingly tolerant of less than optimal nitrogen levels. For example, they found that in five species kept in captivity, no reduction of growth or feeding was noted at a pH as low as 7.5, salinities in the range of 32 to 38 ppt, and both ammonia and nitrite in concentrations of 0.2 ppm on a long-term basis. Similarly, they reported that nitrate concentrations up to 500 ppm did not seem to affect growth or feeding much, if any. However, they mentioned that nitrate concentrations above 100 ppm may affect reproduction. While we are not suggesting that you subject your octopus to these high levels of nitrogen compounds, we do want to make the point that these animals are more hardy than many have believed.
Octopuses and many invertebrates are sensitive to excess trace levels of heavy metals such as copper. Copper can be introduced into marine systems in several ways. It can leach out of copper pipes and into tap water used to make saltwater. Many fish medications are copper based and will quickly kill invertebrates such as octopuses. Octopuses are also sensitive to low dissolved oxygen concentrations. The common octopus will die if the oxygen concentration falls below 0.25 percent (2.5 ml/l). For reference, 100 percent oxygen-saturated seawater would contain approximately 0.5 to 0.7 percent (5 to 7 ml/l) oxygen in a normal aquarium. The decomposition of excessive organics, the oxidation of ammonia to nitrite, and the oxidation of nitrite to nitrate will lower the dissolved oxygen level in a closed system. While excessive amounts of these waste products may not be directly toxic, their breakdown may have a detrimental effect on respiration.
Preventing escape from the aquarium is crucialâthe importance of keeping an octopus safely confined can't be overemphasized. For over 2000
years, records have been written of octopuses leaving the water. In 330 BCE, Aristotle wrote that octopuses are the only cephalopods that go on dry land. Pliny the Elder, in his Naturalis Historia (circa AD 77), wrote of a giant octopus, supposedly over 600 lb. (300 kg), that stole salted fish from villagers on the shore. Since these first records, there have been countless stories of octopuses getting into and out of all sorts of trouble by leaving the water and escaping their confines.
In more recent times, there have been a number of credible and more than a few incredible stories of captive octopuses escaping their confines. In 1875, Henry Lee wrote extensively about his experiences with octopuses in the first British public aquarium at Brighton. He related the oft-cited story of an octopus that crawled out of the water over to a nearby tank to eat lumpfish before returning to its own tank. Brighton aquarists were left with a puzzling mystery of the disappearing lumpfish, until one morning when the octopus was discovered in the lumpfish tank. This story was popularly received in newspapers of the time and even inspired a poem. Various versions of this story still permeate popular culture as urban myths. In this case, there is a seed of truth to the myths since octopuses do move from their homes to forage for food.
A more recent example of an octopus getting into trouble occurred when a giant Pacific octopus kept at the Cabrillo Marine Aquarium in San Pedro, California, pulled out the plastic pipe serving as a water drain, and died after the water drained out of the tank. Some octopus escape stories are amusing. One of our colleagues was transporting octopuses from Indonesia in an ice chest as carry-on luggage aboard an airplane when one escaped, causing a ruckus among the passengers. The octopus was rescued and survived.
In captivity, some octopus species are more likely to escape than others. We surveyed thirty-eight scientists and public aquarists to find out which species of octopuses were most likely to escape. They reported that common octopuses and giant Pacific octopuses are very prone to escaping. High likeliness to escape was also reported for Caribbean reef octopuses; two-spot and blue-ringed octopuses are less likely to escape. However, we have heard of cases where even these species have escaped captivity. In the case of blue-ringed octopuses, the results could be deadly and not just for the octopus. Keeping captive octopuses contained is critical for their welfare. In the story from the Brighton Aquarium, the octopus returned to its tank every night. However, they often do not make it back to a marine environment. If out of the water too long, they will become desiccated and die. To escape, an octopus must be able to hold onto a surface and climb out or find a hole large enough to squeeze through. Octopuses' suckers are amazingly strong: a single sucker with a ¼ in. (6 mm) diameter can hold 5.2 oz. (148 g). An octopus can easily pull many times its body weight and can walk straight up a wall.

Escape Artists
In 1993, I was returning from a research cruise to Dry Tortugas National Park in the Florida Keys, when we stopped for the night in Key West. I intended to catch an octopus but didn't have buckets with lids; I did have several buckets and my trusty tropical fish collecting net. A friend came along with me and we headed for the rock jetty that protected the marina from waves. Within 20 minutes, we found an octopus. This one was a good-sized (baseball sized, all balled up, and 2 ft. [0.6 m] across, stretched out) Caribbean reef octopus that had been through a lot. Most of her arms were in various states of regrowth and she had scars. When I first caught her, she quickly found a tiny hole in the dive net and was out. I caught her again and she escaped again. The third time I caught her, I quickly closed the hole with my hand.
She was placed in a bucket with 1 gal. (3 l) of water. Not having a lid, I filled the other bucket two-thirds full of water and slid it into the first bucket to cover it. I carefully inspected this setup, knowing octopuses' strength and ability to escape. The water in the top bucket must have weighted at least 20 lb. (9 kg) (and the crack between the two buckets was no larger than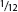 in. (13 mm) at the most, much too small for the beak of an octopus that size to get through. My friend said years later that at the time he thought I was being overly careful in my precautions.
in. (13 mm) at the most, much too small for the beak of an octopus that size to get through. My friend said years later that at the time he thought I was being overly careful in my precautions.
We looked for a smaller octopus with full arms that night but didn't see any more cephalopods. About an hour later, we returned to the buckets. The octopus was gone, escaped even before we got her home. To escape from those buckets, she must have pushed the bucket of water up to enlarge the crack and held it there while she escaped. We would have loved to see how she managed to do that.
I can't help but think that this is exactly what evolution has selected in octopuses, a creature designed to use its flexible body, tricks, and smarts to avoid predation from vertebrates. They are truly masters at getting into and out of tight spaces. Many of us have observed octopuses leave the water in the wild; this is true for giant Pacific octopuses and red octopuses in the northeast Pacific and the Caribbean reef octopus and the common octopus in the Caribbean. Norman reports that
Octopus alpheus
will leave the water to crawl between tide pools. Leaving the water is a normal and natural behavior for some species although it can be lethal in captivity.
âJames B. Wood
