E=mc2 (18 page)
Authors: David Bodanis

The flash of light from the explosion over Hiroshima in 1945 reached the orbit of the moon. Some of it bounced back to Earth; much of the rest continued onward, traveling all the way to the sun, and then indefinitely beyond. The glare would have been viewable from Jupiter.
In the perspective of the galaxy, it was the most insignificant flicker.
Our sun, alone, explodes the equivalent of many million such bombs every second. For E=mc
2
does not apply just on Earth. All the scrambling commandos and anxious scientists and cold-eyed bureaucrats: all that is but a drop, the slightest added whisper, in the enormous powerful onrushing of the equation.
Einstein and other physicists had long recognized this; it was just a quirk that the accelerated technology and pressures of wartime had led to the equation's first applications being focused on weaponry. In this section of the book we switch to those wider views; lifting away from earthly technology, and showing how the equation's sway extends throughout the universe: controlling everything from how the first stars ignited, to how life will end.
. . .
Ever since the discovery of radioactivity in the 1890s, researchers had suspected that uranium or a similar fuel might be operating in the broader universe, and in particular, in our sun to keep it burning. Something that powerful was needed, because Darwin's insights as well as findings in geology had shown that Earth must have been in existence—and warmed by the sun—for billions of years. Coal or other conventional fuels would not be strong enough to do that.
Unfortunately, though, astronomers couldn't find any signs of uranium in the sun. Every element gives off a distinctive visual signal, and the optical device called the spectroscope (for it breaks apart the "spectrum") allows them to be identified. But point a spectroscope at the sun, and the signals are clear: there is no uranium or thorium or other known radioactively glowing element up there.
What did seem to leap out, in readings from distant stars as well as our own sun, was that there was always iron inside them: lots and lots of metallic bulky iron. By the time Einstein was finally able to leave the patent office, in 1909, the best evidence was that the sun was about
66
percent pure iron.
This was a disheartening result. Uranium could pour out energy in accord with E=mc
2
, because the uranium nucleus is
so
large and overstuffed that it barely holds together. Iron is different. Its nucleus is one of the most perfect and most stable imaginable. A sphere made of iron—even if it was molten or gaseous or ionized iron—could not pour out heat for thousands of millions of years.
Suddenly the vision of using E=mc
2
and related equations to explain the whole universe was blocked. Astronomers could just look past the top of the atmosphere, to the great spaces and waiting suns beyond us, and wonder.
The individual who broke that barrier—letting E=mc
2
slip the surly bonds of Earth—was a young Englishwoman named Cecilia Payne, who loved seeing how far her mind could take her. Unfortunately, the first teachers she found at Cambridge when she entered in 1919 had no interest in such explorations. She switched majors, and then switched again, which led to her reading up on astronomy, and when Payne decided on
anything,
the effects were impressive. She terrified the night assistant at the university's telescope her first night there, after she'd been reading for only a few days. He "fled down the stairs," she recalled, "gasping: 'There's a woman out there asking questions.'" But she wasn't put off, and a few weeks later she described another such incident: "I bicycled up to the Solar Physics Observatory with a question in my mind. I found a young man, his fair hair tumbling over his eyes, sitting astride the roof of one of the buildings, repairing it. CI have come to ask,' I shouted up at him, 'why the Stark effect is not observed in stellar spectra.'"
This time her subject did not flee. He was an astronomer himself, Edward Milne, and they became friends. Payne tried to pull her arts student friends into her astronomical excitements, and even though they might not have understood much of what she was saying, she was the sort of person others like being around. Her rooms at Newnham College were almost always crowded. A friend wrote: ". . . when safely lying on her back on the floor (she despises armchairs), she will talk of all things under the sun, from ethics to a new theory of making cocoa."
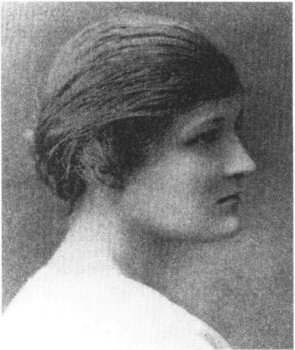
Cecilia Payne
THE PRINCIPAL AND FELLOWS OF NEWNHAM COLLEGE, CAMBRIDGE, ENGLAND
Rutherford was teaching at Cambridge by then, but didn't know what to do with Payne. With men he was bluff and friendly, but with women he was bluff and pretty much a thug. He was cruel to her at lectures, trying to get all the male students to laugh at this one female in their midst. It didn't stop her from going—she could hold her own with his best students in tutorials— but even forty years later, retired from her professorship at Harvard, she remembered the rows of braying young men, nervously trying to do what their teacher expected of them.
But Arthur Eddington, a quiet Quaker, was also at the university, and he was happy to take her on as a tutorial student. Although his reserve never lifted—tea with students was generally in the presence of his elderly unmarried sister—the twenty-year-old Payne picked up Eddington's barely stated awe at the potential power of pure thought.
He liked to show how creatures who lived on a planet entirely shrouded in cloud would be able to deduce the main features of the unseen universe above them. There would have to be glowing spheres out there, he imagined them reasoning, for the original gas clouds floating in space would gradually form dense enough clumps to start nuclear reactions inside and light up—they would become suns. These glowing spheres would be dense enough to pull planets swinging around them. If the beings on the mythical planet ever did find that a sudden wind had blown an opening in their clouds, when they looked up they'd see a universe of glowing stars, with circling planets, just as they'd expected.
It was exhilarating to think that someone on Earth might solve the problem of how to deal with all the iron in the sun, and so be able actually to work out Eddington's vision. When Eddington first assigned Payne a problem on stellar interiors, which might at least start to achieve this, "the problem haunted me day and night. I recall a vivid dream that I was at the center of [the giant star] Betelgeuse, and that, as seen from there, the solution was perfectly plain; but it did not seem so in the light of day."
But even with this kind man's backing, a woman couldn't do graduate work in this field in England, so she went to Harvard, and there blossomed even more. She switched from her heavy woolen clothing to the lighter fashions of 1920s America; she found a thesis adviser, Harlow Shapley, an up-and-coming astrophysicist; she loved the liberty she found in the student dorms, and the fresh topics in the university seminars. She was bursting with enthusiasm.
And that could have blocked everything. Raw enthusiasm is dangerous for young researchers. If you're excited by a new field—keen to join in with what your professors and fellow students are doing—that usually means you'll be trying to fit in with their approaches. But students whose work stands out usually have had some reason to avoid this, and keep a critical distance. Einstein didn't especially respect his Zurich professors: most, he thought, were drudges, who never questioned the foundations of their teaching. Faraday couldn't be content with explanations that left out the inner feelings of his religion; Lavoisier was offended at the vague, inexact chemistry handed down by his predecessors. For Payne, some of her needed distance came from getting to know her fun fellow Ivy League students a little better. Shortly after arriving: "I expressed to a friend that I liked one of the other girls in the House where I lived at Radcliffe College. She was shocked: 'But she's a Jew!' was her comment. This frankly puzzled me. . . . I found the same attitude towards those of African descent."
She also got a glimpse of what was going on in the back rooms at the Observatory. In 1923, the word
computer
did not mean an electrical machine. It meant people whose sole job was to compute. At Harvard, it was applied to ranks of slump-shouldered spinsters in those back rooms. A few
of
them had once had first-rate scientific talent ("I always wanted to learn the calculus," one said, "but [the director] did not wish it"), yet that was usually long since crushed out of them, as they were kept busy measuring star locations, or cataloging volumes of previous results. If they got married they could be fired; if they complained of their low salaries, they would be fired as well.
Lise Meitner had had her problems in getting started in research in Berlin, but there was nothing like this desolate, life-crushing sexism. A few of the Harvard "computers," in several decades of bent-back work, succeeded in measuring over 100,000 spectral lines. But what it meant, or how it fit in with the latest developments in physics, was almost always not for them to understand.
Payne was not going to be pushed into their ranks. Spectroscope readings can be ambiguous where they overlap. Payne began to wonder how much the way her professors broke them apart depended on what they already had in mind. For example, let the reader note the following letters very well, and then try to read them:
n o t e
v e r y
o n e w
e l l g
e t i t
It's not easy. But if you start reading it instead as "Not everyone . . . " then it leaps out. What Cecilia Payne decided on, there in 1920s Boston, was a Ph.D. project that would let her confirm and further develop a new theory about how to build up spectroscope interpretations. Her work was more complicated than our example above, for spectroscope lines from the sun will always include fragments of several elements; there are distortions from the great temperature as well.
An analogy can show what Payne did. If astronomers are convinced there's going to be lots of iron in the sun (which seemed fair for there was so much iron on Earth and in asteroids), there'd be only one way to read an ambiguous string of lines from a spectroscope. If they came out, for example, as:
t h e y s a i d i r o n a g a i e n
you'd parse it to read:
t h e y s a i d
I r o n
a g a i e n
and there'd be no need to worry too much about the odd spelling
of agaien.
The extra
e
could be a fault in the spectroscope, or some odd reaction on the sun, or just a fragment that was slipped in from some other element. There's always something that doesn't fit. But Payne kept an open mind. What if it was really trying to communicate:
t
H
e
y
s a i
d
i
r o
n a
g
a i
e n
She went through the spectroscope lines over and over again, checking for these ambiguities. Everyone had boosted the lines one way, to make it read as if they were for iron. But it wasn't too much of a stretch to boost them differently, so that they read hydrogen, not iron.
Even before Payne finished her doctorate, her results began to spread in gossip among astrophysicists. While the old explanation of the spectroscope data had been that the sun was two-thirds iron or more, this young woman's interpretation was that it was over 90 percent
hydrogen,
with most of the rest being the nearly as lightweight helium. If she was right, it would change what was understood about how stars burn. Iron is so stable that no one could imagine it transformed through E=mc
2
to generate heat in our Sun. But who knew what hydrogen might do?
The old guard knew. Hydrogen would do nothing. It wasn't there, it couldn't be there; their careers—all their detailed calculations, and the power and patronage that stemmed from it—depended on iron being what was in the sun. After all, hadn't this female only picked up the spectroscopic lines from the sun's outer atmosphere, rather than its deep interior? Maybe her readings were simply confused by the temperature shifts or chemical mixes there. Her thesis adviser declared her wrong, and then
his old
thesis adviser, the imperious Henry Norris Russell, declared her wrong, and against him there was very little recourse. Russell was an exceptionally pompous man, who would never accept he could be wrong—and he also controlled most grants and job appointments in astronomy on the East Coast.
For a while Payne tried to fight it anyway: repeating her evidence; showing the way her hydrogen interpretation was just as plausible in the spectroscope lines as the iron ones; even more, the way new insights—the latest in European theoretical physics—were suggesting a way hydrogen really could power the sun. It didn't matter. She even tried reaching out to Eddington, but he withdrew, possibly out of conviction, possibly out of caution before Russell—or possibly just from a middle-aged bachelor's fear of a young woman turning to him with emotion. Her friend from her student days at the Cambridge Solar Physics Observatory, the young fair-haired Edward Milne, was by now an established astronomer, and did try to help, but he didn't have enough power. Letters were exchanged between Payne and Russell, but if she wanted
to
get her research accepted she'd have to recant. In her own published thesis she had to insert the humiliating line: "The enormous abundance [of hydrogen] . . . is almost certainly not real."
