Full House (19 page)

The logic of this argument is sound, but two strong reasons suggest (though not all the evidence is yet in) that the proposition is empirically false. (I shall summarize the two reasons here and provide more details later in this chapter.) First, while I know of no proven bias for rightward motion under natural selection—a mechanism that yields only local adaptation to changing environments, not general progress—a good case can be made for leftward bias because parasitism is such a common evolutionary strategy, and parasites tend to be anatomically more simplified than their free-living ancestors. (Ironically, then, the full system of increasing right skew for the whole might actually be built with a slight bias toward decreased complexity in individual lineages!) Second, several paleontologists are now studying this issue directly by trying to quantify the elusive notion of progress and then tracing the changing spread of their measure in the history of individual lineages. Only a few studies have been completed so far, but current results show no rightward bias, and therefore no tendency to progress in individual lineages.
7. Even a parochial decision to focus on the right tail alone will not yield the one, most truly desired conclusion, the psychological impetus to our yearning for general progress—that is, the predictable and sensible evolution to domination of a creature like us, endowed with consciousness. We might adopt a position of substantial retreat from an original claim for general progress, but still a bastion of defense for what really matters to us. That is, we might say, "Okay, you win. I understand your point that the evidence of supposed progress, the increasing right skew of life’s bell curve, is only an epiphenomenal tail that cannot wag the entire dog—and that life’s full house has never moved from its modal position. But I am allowed to be parochial. The right tail may be small and epiphenomenal, but I love the right tail because I dwell at its end—and I want to focus on the right tail alone because this little epiphenomenon is all that matters to me. Even you admit that the right tail had to arise, so long as life expanded. So the right tail had to develop and grow—and had to produce, at its apogee, something like me. I therefore remain the modern equivalent of the apple of God’s eye: the predictably most complex creature that ever lived."
Wrong again, even for this pitifully restricted claim (after advancing an initial argument for intrinsic directionality in the basic causal thrust of all evolution). The right tail had to exist, but the actual composition of creatures on the tail is utterly unpredictable, partly random, and entirely contingent —not at all foreordained by the mechanisms of evolution. If we could replay the game of life again and again, always starting at the left wall and expanding thereafter in diversity, we would get a right tail almost every time, but the inhabitants of this region of greatest complexity would be wildly and unpredictably different in each rendition—and the vast majority of replays would never produce (on the finite scale of a planet’s lifetime) a creature with self-consciousness. Humans are here by the luck of the draw, not the inevitability of life’s direction or evolution’s mechanism.
In any case, little tails, no tails, or whoever occupies the tails, the outstanding feature of life’s history has been the stability of its bacterial mode over billions of years!
The Multifariousness of the Modal Bacter
My interest in paleontology began in a childhood fascination with dinosaurs. I spent a substantial part of my youth reading the modest literature then available for children on the history of life. I well remember the invariant scheme used to divide the fossil record into a series of "ages" representing the progress that supposedly marked the march of evolution: the "Age of Invertebrates," followed by the Age of Fishes, Reptiles, Mammals, and, finally, with all the parochiality of the engendered language then current, the "Age of Man."
I have watched various reforms in this system during the past forty years (though see chapter 2, for persisting use of the old scheme). The language police, of course, would never allow an Age of Man anymore, so we could, at best and with more inclusive generosity, now specify an "age of humans" or an "age of self-consciousness." But we have also come to recognize, with even further inclusive generosity, that one species of mammals, despite our unbounded success, cannot speak adequately for the whole. Some enlightened folks have even recognized that an "age of mammals" doesn’t specify sufficient equity—especially since mammals form a small group of some four thousand species, while nearly a million species of multicellular animals have been formally named. Since more than 80 percent of these million are arthropods, and since the great majority of arthropods are insects, these same enlightened people tend to label modern times as the "age of arthropods."
Fair enough, if we wish to honor multicellular creatures—but we are still not free of the parochialism of our scale. If we must characterize a whole by a representative part, we certainly should honor life’s constant mode. We live now in the "Age of Bacteria." Our planet has always been in the "Age of Bacteria," ever since the first fossils—bacteria, of course— were entombed in rocks more than three and a half billion years ago.
On any possible, reasonable, or fair criterion, bacteria are—and always have been—the dominant forms of life on earth. Our failure to grasp this most evident of biological facts arises in part from the blindness of our arrogance, but also, in large measure, as an effect of scale. We are so accustomed to viewing phenomena of our scale—sizes measured in feet and ages in decades—as typical of nature. Individual bacteria lie beneath our vision and may live no longer than the time I take to eat lunch, or my grandfather spent with his evening cigar. But then, who knows? To a bacterium, human bodies might appear as widely dispersed, effectively eternal (or at least geological), massive mountains, fit for all forms of exploitation, and fraught with little danger unless a bolus of imported penicillin strikes at some of the nasty brethren.
Consider just some of the criteria for bacterial domination:
TIME. I have already mentioned the persistence of bacterial rule. The fossil record of life begins with bacteria, some 3.5 to 3.6 billion years ago. About half the history of life later, the more elaborate eukaryotic cell makes a first appearance in the fossil record—about 1.8 to 1.9 billion years ago by best current evidence. The first multicellular creatures—marine algae—enter the stage soon afterward, but these organisms bear no genealogical relationship to our primary (if admittedly parochial) interest in this book: the history of animal life. The first multicellular animals do not enter the fossil record until about 580 million years ago—that is, after about five-sixths of life’s history had already passed. Bacteria have been the stayers and keepers of life’s history.
Moreover, bacteria do not record their history of Precambrian domination as invisible dots in rocks. Rather, they shaped their environments, and left their sedimentary records, in highly visible form—even though no multicellular animals then lived to view the effect. The fossil record of ancient bacteria consists largely of stromatolites—complexly concentric and laminated layers, often looking like a head of cabbage in cross-section (see Figure 30). These sizable structures are not bacteria themselves, but layers of sediments trapped and bound by mats of bacterial cells. Most stromatolites formed near the tide lines, and were constantly desiccated and regrown during fluctuations in sea level—thus leading to large, vertical piles of wavy layers. Stromatolites still exist, but now can form only in unusual environments devoid of the multicellular animals that happily feed on such organisms and therefore prevent their formation in most places. But no potential feeders lived in these early years, during most of life’s history, and stromatolites must have covered appropriate habitats throughout the planet.

FIGURE 30 Modern stromatolites—layers of sediment trapped and bound by prokaryotic cells.
INDESTRUCTIBILITY. Let us make a quick bow to the flip side of such long domination—to the future prospects that match such a distinguished and persistent past. Bacteria have occupied life’s mode from the very beginning, and I cannot imagine a change of status, even under any conceivable new regime that human ingenuity might someday impose upon our planet. Bacteria exist in such overwhelming number, and such unparalleled variety; they live in such a wide range of environments, and work in so many unmatched modes of metabolism. Our shenanigans, nuclear and otherwise, might easily lead to our own destruction in the foreseeable future. We might take most of the large terrestrial vertebrates with us—a few thousand species at most. We surely cannot extirpate 500,000 species of beetles, though we might make a significant dent. I doubt that we could ever substantially touch bacterial diversity. The modal organisms cannot be nuked into oblivion, or very much affected by any of our considerable conceivable malfeasances.
TAXONOMY. The history of classification for the basic groups of life is one long tale of decreasing parochialism and growing recognition of the diversity and importance of single-celled organisms, and other "lower" creatures. Most of Western history favored the biblically sanctioned twofold division of organisms into plants and animals (with a third realm for all inorganic substances—leading to the old taxonomy of "animal, vegetable, or mineral" in such venerable games as Twenty Questions). This twofold division produced a host of practical consequences, including the separation of biological research into two academic departments and traditions of study: zoology and botany. Under this system, all single-celled organisms had to fall into one camp or the other, however uncomfortably, and however tight the shove of the shoehorn. Thus, paramecia and amoebae became animals because they move and ingest food. Photosynthesizing unicells, of course, became plants. But what about photosynthesizers with mobility? And, above all, what about the prokaryotic bacteria, which bear no key feature suggesting either allocation? But since bacteria have a strong cell wall, and because many species are photosynthetic, bacteria fell into the domain of botany. To this day, we still talk about the bacterial "flora" of our guts.
By the time I entered high school in the mid-1950s, expansion and enlightenment had proceeded far enough to acknowledge that unicells could not be so divided by criteria of the multicellular world, and that single-celled organisms probably deserved a separate kingdom of their own, usually called Protista.
Twelve years later, as I left graduate school, even greater respect for the unicells had led to further proliferation at the "lower" end. A "five kingdom" system was now all the rage (and has since become canonical in textbooks)—with the three multicellular kingdoms of plants, fungi, and animals in a top layer (representing, loosely, production, decomposition, and ingestion as basic modes of life); the eukaryotic unicells, or Kingdom Protista, in a middle layer; and the prokaryotic unicells, or Kingdom Monera, representing bacteria and "blue-green algae," on a bottom rung. Most proponents of this system recognized the gap between prokaryotic and eukaryotic organization—that is, the transition from Monera to Protista—as the fundamental division within life, thus finally granting bacteria their measure of independent respect, if only as a bottom tier.
A decade later, starting in the mid-1970s, development of techniques for sequencing the genetic code finally gave us a key for mapping evolutionary relationships among bacterial lineages. (We know how to use anatomy for drawing genealogical trees of multicellular creatures more familiar to us—so we employ the internal skeleton of vertebrates, the external carapace of arthropods, and the multiplated test and radial symmetry of echinoderms to identify major evolutionary groups. But we are so ignorant of the bacterial world that we couldn’t identify proper genealogical divisions—and we therefore tended to dump all bacteria together into a bag of little unicellular blobs, rods, and spirals. Yet we should have suspected deep divisions, far more extensive than those separating lines of multicellular animals—if only because bacteria have inhabited the planet for so long.)
As nucleotide sequences began to accumulate for key segments of bacterial genomes, a fascinating and unsuspected pattern emerged—and has grown ever stronger with passing years and further accumulation of evidence. This group of supposed primitives, once shoved into one small bag for their limited range of overt anatomical diversity, actually includes two great divisions, each far larger in scope (in terms of genomic distinction and variety) than all three multicellular kingdoms (plants, animals, and fungi) combined! Moreover, one of these divisions seemed to gather together, into one grand sibship, most of the bacteria living in odd environments and working by peculiar metabolisms under extreme conditions (often in the absence of oxygen) that may have flourished early in the earth’s history—the methanogens, or methane producers; the tolerators of high salinities, the halophiles; and the thrivers at temperatures around the boiling point of water, the thermophiles.
These first accurate genealogical maps led to the apparently inescapable conclusion that two grand kingdoms, or domains, must be recognized within the old kingdom Monera—Bacteria for most conventional forms that come to mind when we contemplate this category (the photosynthesizing blue-greens, the gut bacteria, the organisms that cause human diseases and therefore become "germs" in our vernacular); and Archaea for the newly recognized coherence of oddballs. By contrast, all eukaryotic organisms, the three multicellular kingdoms as well as all unicellular eukaryotes, belong to a third great evolutionary domain, the Eucarya.
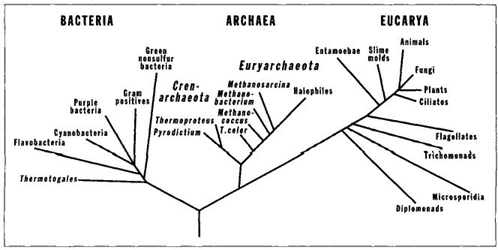
FIGURE 31 Life’s evolutionary tree, showing two prokaryotic domains and only one eukaryotic domain, with plants, animals, and fungi as small twigs at an extreme of the eukaryotic domain.
The accompanying chart (Figure 31), from the work of Carl Woese, our greatest pioneer in this new constitution of life, says it all, with the maximally stunning device of a revolutionary picture. We now have a system of three grand evolutionary domains—Bacteria, Archaea, and Eucarya— and two of the three consist entirely of prokaryotes: that is, "bacteria" in the vernacular, the inhabitants of life’s constant mode. Once we place two-thirds of evolutionary diversity at life’s mode, we have much less trouble grasping the centrality of this location, and the constant domination of life by bacteria. For example, the domain of Bacteria, as presently defined, contains eleven major subdivisions, and the genetic distance between any pair is at least equal to the average separation between eukaryotic kingdoms such as plants and animals (Fuhrman, McCallum, and Davis, 1992).
Note, by contrast and in closing, the restricted domain of all three multicellular kingdoms. On this genealogical chart for all life, the three multicellular kingdoms form three little twigs on the bush of just one among three grand domains of life. Quite a change in one generation—from my parents’ learning that everything living must be animal or vegetable, to the icon of my mature years: the kingdoms Animalia and Plantae as two little twigs amid a plethora of other branches on one of three bushes—with both other bushes growing bacteria, and only bacteria, all over.
UBIQUITY. The taxonomic criterion (Figure 31), while impressive, does not guarantee bacterial domination—and for a definite reason common to all genealogical schemes. Bacteria form the root of life’s entire tree. For the first 2 billion years or so, about half of life’s full history, bacteria alone built the tree of life. Therefore all multicellular creatures, as late arrivers, can only inhabit some topmost branches; the roots and trunk must be exclusively bacterial. This geometry does not make the case for calling our modern world an "Age of Bacteria" because the roots and trunk might now be atrophied, with only the multicellular branches flourishing. We need to show not only that bacteria build most of life’s tree, but also that these bacterial foundations remain strong, healthy, vigorous, and fully supportive of the minor superstructure called multicellular life. Bacteria, indeed, have retained their predominant position, and hold sway not only by virtue of a long and illustrious history, but also for abundant reasons of contemporary vigor. Consider two aspects of ubiquity:
