Hen’s Teeth and Horse’s Toes (31 page)
Read Hen’s Teeth and Horse’s Toes Online
Authors: Stephen Jay Gould

We must beware of reading too much into this arresting conclusion since, as Raup and Sepkoski remind us, biases of the fossil record must always be suspected as an artificial cause of such patterns. For example, probability of preservation increases for fossils in progressively younger rocks (greater geographic extent of sediments, less opportunity for destruction of fossils by subsequent heat and pressure). Perhaps older families seem to live for shorter times simply because records of their early or late appearance are not preserved. But if this pattern does reflect a biological reality, then it suggests that modern families are more resistant to extinction and that the total rise in life’s diversity may be a result of this increasing general hardiness.
Still, heroic though the fight may be (in inappropriate metaphor), organisms cannot win. The rate of familial extinction may have been cut in half during life’s recorded history, but no species is immortal, and all must ultimately perish. The perfection of immediate adaptation is no protection against massive fluctuations of environment that inevitably, in the course of millions of years, affect every corner of the globe. Since Darwinian processes can only improve local adaptations, and since species cannot reckon the future (with one interesting and imperfect exception), all will eventually perish, leaving as potential patrimony only the altered descendants that may branch off from them.
I was in York Cathedral this spring, where I found the essence of this theme expressed in charming doggerel on the seventeenth-century tomb of one William Gee. Sir William, it seems, lived such a blameless life that if God wished to confer immortality upon anyone, His candidate had surely come forth. But Sir William died, so God must not entertain this option:
If universal learning, language, law
Pure piety, religion’s reverend awe,
Fair friends, fair issue: if a virtuous wife,
A quiet conscience, a contented life,
The clergy’s prayers or the poor man’s tears
Could have lent length to man’s determined years.
Sure as the fate, which for our fault we fear,
Proud death had ne’er advanced this trophy here.
In it behold thy doom, thy tomb provide.
Sir William Gee had all these pleas, yet died.
(I have modernized spelling and punctuation, but not words or grammar. In line 8, read “would have” for the old subjunctive “had.” Sir William’s “issue” are his kids, so perhaps the family line survives.)
Inevitabilities should never be depressing. An old philosophical tradition, dating at least to Spinoza, proclaims that freedom is the recognition of necessity. If we respect intellect, true freedom must come from learning the ways of the world—what can be changed and what cannot—and by shaping a gutsy life accordingly. Besides, if species lived forever, we would have no science of paleontology, and I might have become a fireman after all.
EACH YEAR
, professional scientists scan thousands of titles, read hundreds of abstracts, and study a few papers in depth. Since titles are the commonest, and usually the only, form of contact between writers and potential readers in the great glut of scientific literature, catchy items are appreciated and remembered, but unfortunately rare. Every scientist has his favorite title. Mine was coined by paleontologist Albert E. Wood in 1957: “What, If Anything, Is a Rabbit?”
Wood’s question may have been wry, but his conclusion was ringing: rabbits and their relatives form a coherent, well-defined order of mammals, not particularly close to rodents in evolutionary descent. I was reminded of Wood’s title recently when I read a serious challenge to the integrity of a personal favorite among mammals: the zebra. Now don’t get too agitated. I am not trying to turn the world of received opinion upside down. Striped horses manifestly exist. But do they form a true evolutionary unit? With “Stripes Do Not a Zebra Make”—a quite respectable title in its own right—Debra K. Bennett has forced us to extend Wood’s question to another group of mammals. What, if anything, is a zebra?
Since evolutionary descent is our criterion for biological ordering, we define groups of animals by their genealogy. We do not join together two distantly related groups because their members have independently evolved some similar features. Humans and bottle-nosed dolphins, for example, share the pinnacle of brain size among mammals. But we do not, for this reason, establish the taxonomic group Psychozoa to house both species—for dolphins are more closely related by descent with whales, and humans with apes. We follow the same principles in our own genealogies. A boy with Down’s syndrome is still his parents’ son and not, by reason of his affliction, more closely related to other Down’s children, no matter how long the list of similar features.
The potential dilemma for zebras is simply stated: they exist as three species, all with black-and-white stripes to be sure, but differing notably in both numbers of stripes and their patterns. (A fourth species, the quagga, became extinct early in this century; it formed stripes only on its neck and forequarters.) These three species are all members of the genus
Equus
, as are true horses, asses, and donkeys. (In this essay, I use “horse” in the generic sense to specify all members of
Equus
, including asses and zebras. When I mean Old Dobbin or Man o’ War, I will write “true horses.”) The integrity of zebras then hinges on the answer to a single question: Do these three species form a single evolutionary unit? Do they share a common ancestor that gave rise to them alone and to no other species of horse? Or are some zebras more closely related by descent to true horses or to asses than they are to other zebras? If this second possibility is an actuality, as Bennett suggests, then horses with black-and-white stripes arose more than once within the genus
Equus
, and there is, in an important evolutionary sense, no such thing as a zebra.
But how can we tell, since no one witnessed the origin of zebra species (or at least australopithecines weren’t taking notes at the time), and the fossil record is, in this case, too inadequate to identify events at so fine a scale. During the past twenty years, a set of procedures has been codified within the science of systematics for resolving issues of this kind. The method, called cladistics, is a formalization of procedures that good taxonomists followed intuitively but did not properly express in words, leading to endless quibbling and fuzziness of concepts. A clade is a branch on an evolutionary tree, and cladistics attempts to establish the pattern of branching for a set of related species.
Cladistics has generated a fearful jargon, and many of its leading exponents in America are among the most contentious scientists I have ever encountered. But behind the names and nastiness lies an important set of principles. Still the clear formulation of principles does not guarantee an unambiguous application in each case—as we shall see for our zebras.
I believe that we can get by with just two terms from the bounty offered by cladists. Two lineages sharing a common ancestor from which no other lineage has sprung form a
sister group
. My brother and I form a sister group (pardon the confusion of gender) because he is my only sib and neither of my parents had any other children.
Cladists attempt to construct hierarchies of sister groups in order to specify temporal order of branching in evolutionary history. For example: gorillas and chimpanzees form a sister group because no other primate species branched from their common ancestor. We may then take the chimpgorilla sister group as a unit and ask which primate forms a sister group with it. The answer, according to most experts, is us. We now have a sister group with three species, each more closely related to its two partners than to any other species.
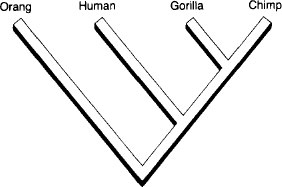
The cladistic pattern of great apes and humans.
REPRINTED FROM NATURAL HISTORY. DRAWING BY JOE LE MONNIER
.
We may extend this process indefinitely to form a chart of branching relationships called a cladogram. But consider just one more step: What primate species is the sister group to the human-chimp-gorilla unit? Conventional wisdom cites the orangutan, and we add it to our cladogram.
This cladogram of “higher” primates contains an interesting implication: there is no such thing as an ape, at least as usually defined. Several species of primates may swing through trees, eat bananas in zoos, and form good prototypes for science fiction of various sorts. But orangs, chimps, and gorillas (the “apes” of our vernacular) are not a genealogical unit because orangs are cladistically more distant from chimps and gorillas than humans are—and we originally defined the term ape to contrast some lesser forms with our exalted state, not to include us!
The zebra problem can also be placed in this context. If the three species of zebras form a sister group (as humans, chimps, and gorillas do on our cladogram), then each is more closely related to its two partners than to any species of horse, and zebras form a true evolutionary unit. But if zebras are like “apes,” and another species of horse lies within the cladogram of zebras (as humans lie within the cladogram of traditional apes), then striped horses may share some striking similarities meriting a common vernacular term (like zebra), but they are not a genealogical unit.
But how do we identify sister groups correctly? Cladists argue that we must search for—and here comes the second term
—shared derived characters
(technically called synapomorphies). Primitive characters are features present in a distant common ancestor; they may be lost or modified independently in several subsequent lineages. We must be careful to avoid primitive characters in searching for common features to identify sister groups, for they spell nothing but trouble and error. Humans and many salamanders have five toes; horses have one. We may not therefore state that humans are more closely related to salamanders than to horses, and that the concept of “mammal” is therefore a fiction. Rather, five toes is an inadmissible primitive character. The common ancestor of all terrestrial vertebrates had five toes. Salamanders and humans have retained the original number. Horses—and whales and cows and snakes and a host of other vertebrates—have lost some or all of their toes.
Derived characters, on the other hand, are features present
only
in members of an immediate lineage. They are unique and newly evolved. All mammals, for example, have hair; no other vertebrate does. Hair is a derived character for the class Mammalia because it evolved but once in the common ancestor of mammals and therefore identifies a true branch on the family tree of vertebrates. Shared derived characters are held in common by two or more lineages and may be used to specify sister groups. If we wish to identify the sister group among tunas, seals, and bobcats, we may use hair as a shared derived character to unite the two mammals and to eliminate the fish.
For zebras, the question then becomes: Are stripes a shared derived character of the three species? If so, the species form a sister group and zebras are a genealogical unit. If not, as Bennett argues, then zebras are a disparate group of horses with some confusing similarities.
The method of cladistics is both simple and sensible: establish sequences of sister groups by identifying shared derived characters. Unfortunately, conceptual elegance does not guarantee ease of application. The rub, in this case, lies in determining just what is or is not a shared derived character. We have some rough guidelines, and some seat-of-the-pants feelings, but no unerring formulas. If derived characters are sufficiently “complex,” for example, we begin to feel confident that they could not have evolved independently in separate lineages and that their mutual presence therefore indicates common descent.
Chimps and gorillas share a set of complex and apparently independent modifications in several of their chromosomes (mostly “inversions,” literally, the turning around of part of a chromosome by breaking, flipping, and reattaching). Since these chromosomal changes are complex and do not seem to represent “easy” modifications so adaptively necessary that separate lineages might evolve them independently, we mark them as shared derived characters present in the common ancestor of chimps and gorillas, and in no other primate. Hence they identify chimps and gorillas as a sister group.
Unfortunately, most derived characters are more ambiguous. They tend to be either easy to construct or else so advantageous that several lineages might evolve them independently by natural selection. Many mammals, for example, develop a sagittal crest—a ridge of bone running along the top of the skull from front to back and serving as an attachment site for muscles. Most primates do not have a sagittal crest, in part because large brains make the cranium bulge and leave neither room nor material for such a structure. But a general rule for scaling of the brain in mammals holds that large animals have relatively smaller brains than relatives of diminished body size (see essays in
Ever Since Darwin
and
The Panda’s Thumb
). Thus, the largest primates have a sagittal crest because their relatively small brains do not impede its formation. (This argument does not apply to the great oddball
Homo sapiens
, with an enormous brain despite its large body.) The largest australopithecine,
Australopithecus boisei
, has a pronounced sagittal crest, while smaller members of the same genus do not. Gorillas also have a sagittal crest, while most smaller primates do not. We would make a great error if, using the sagittal crest as a shared derived character, we united an australopithecine with a gorilla in a sister group and linked other, smaller-bodied australopithecines with marmosets, gibbons, and rhesus monkeys. The sagittal crest is a “simple” character, probably part of the potential developmental repertoire for any primate. It comes and goes in evolution, and its mutual presence does not indicate common descent.
Bennett bases her cladistic analysis of the genus
Equus
on skeletal characters, primarily of the skull. All horses are pretty much alike under the skin, and Bennett has not found any shared derived characters as convincing as the chromosomal similarities of chimps and gorillas. Most of her characters are, by her own admission, more like the sagittal crest—hence the provisional nature of her conclusions.
Bennett argues that the genus
Equus
contains two major cladistic groups—donkeys and asses on one side and true horses and zebras on the other. Thus, zebras pass the first test for consideration as a genealogical unit. Unfortunately (or not, according to your point of view), Bennett claims that they fail the second test. She does identify the Burchell and Grevy zebras (
Equus burchelli
and
E. grevyi
) as a sister group. But in her scheme, the third species, the mountain, or Hartmann, zebra (
E. zebra
) does not join its cousins to form a larger sister group. Instead, the sister species of the mountain zebra is our close compatriot in farm and track, the true horse (
E. caballus
)! Thus, mountain zebras join with true horses before they connect with other zebras. Old Dobbin is inextricably intercalated within the cladogram of zebras—and since he is not a zebra by any definition, then what, if anything, is a zebra?

