In the Beginning Was Information (31 page)
Read In the Beginning Was Information Online
Authors: Werner Gitt
Tags: #RELIGION / Religion & Science, #SCIENCE / Study & Teaching

– mechanical work (energy)
– potential and kinetic energy (energy of rotation and energy of translation)
– the energy of gravitational fields, and of electrical, magnetic, and electromagnetic fields
– heat energy
– electrical energy
– the energy which binds nucleons in atomic nuclei
– chemical energy
– radiation energy of particles (electrons, protons, and neutrons)
– the equivalence of mass and energy
All physical events and processes obey two fundamental principles, known in thermodynamics as the "first law" and the "second law."
The first law:
This important natural law, also known as the "energy law" or the "law of conservation of energy," was first formulated in 1842 by a German physician, Robert Mayer (1814–1879). It states that energy cannot be created in the observable world, neither can it be destroyed. This law is not an axiom, but is derived from experience as are all natural laws (see Theorem N1, paragraph 2.3). In every chemical or physical process, the total energy of the system and its environment, and thus also the total quantity of energy in the universe, remains constant. It is thus impossible to destroy energy or to add to the total quantity of energy. It can only be converted into other forms. Some important consequences of the energy law are:
– Only events which do not change the total amount of energy, can occur in nature. Walter Gerlach (1889–1979), a German physicist, formulated this principle as follows [R1]: "The law of the conservation of energy plays the role of a police commissioner: it decides beforehand whether a line of thought is acceptable or forbidden."
– The impossibility of a perpetual motion machine of the first kind: No machine can be constructed which, after being set in motion, can continue working unless the supply of energy is renewed.
– The different kinds of energy correspond quantitatively, and these energy equivalents can be determined empirically.
The second law:
The first law is only concerned with the conversion between heat energy and mechanical energy or vice versa, without any regard as to whether the conversion actually takes place or not. The second law, however, determines the
direction
of the process. By themselves, all processes run in only one direction, i.e., they are irreversible. We know from experience that if a hot block of copper is put in contact with a cold block in an isolated container, heat will be exchanged; the hot block continues to convey heat to the cold one until an average temperature occurs in both blocks. If two blocks at the same temperature are placed in the container, nothing will happen. It does not contradict the first law when one block becomes warmer and the other one cooler, as long as there is no overall loss or gain of heat.
The second law provides us with a criterion for predicting the direction of a given energy process. An abstract though quite meaningful concept — entropy
S
— is required for a mathematical formulation of this law. Entropy is a quantifiable value which changes whenever heat is converted. In its briefest form, the second law can be expressed as
dS
≥ 0 (for closed systems). The following conclusions can then be drawn:
– Entropy cannot be destroyed, but it can be produced.
– It is impossible to construct a periodically functioning machine which does nothing else but deliver useful work by cooling a single reservoir of heat. This means, for example, that the heat content of the sea cannot be used for propelling a ship.
– Heat cannot by itself flow from a cooler body to a warmer one (R. Clausius, 1850).
– It is impossible to build a
perpetual motion machine of the second kind:
It never happens in nature that an automatic process can let the amount of entropy decrease with no other effect.
The following formulation was first proposed by J. Meixner [M2]: "In the gigantic factory of natural processes, the function of manager is taken over by the production of entropy, because it prescribes the direction and the kinds of the events of the entire industry. The energy principle only plays the role of accountant, being responsible for the balance between what should be and what is."
The ability of a system to perform useful work:
This is an important concept, since work (mechanical effort) can be completely converted into heat. The reverse process, the complete conversion of heat into useful work is theoretically impossible. This asymmetry is a primary result of the second law. In addition, the second law asserts that closed systems tend toward a state where the usable energy is a minimum, and the entropy becomes a maximum. The change in the amount of entropy indicates whether a process is reversible or not. The better a process can prevent an increase in entropy, the more useful energy can be produced. Potential and kinetic energy, as well as electrical energy, can be arbitrarily converted into one another in such a way that the process is very nearly completely reversible and can thus produce a maximum amount of useful work.
On the other hand, heat energy can only be partially converted into mechanical work or into some other form of energy. It is impossible to convert more than a certain fraction of the supplied heat energy, as given by the formula h = (
T
2
-
T
1
)/
T
2
for an ideal Carnot machine (a reversible Carnot cycle; see also paragraph 2.5). This thermodynamically possible amount of useful energy is known by a distinctive name — exergy. The fact that it is impossible to obtain more work from a heat engine than allowed by
η
C
follows directly from the second law.
Living organisms have a greater efficiency (= useful mechanical work obtained from a given energy input) than the maximum thermal efficiency allowed by the second law. This does not contradict this natural law, but indicates that the Creator has endowed body muscles with the capacity to convert chemical energy directly into mechanical work, and do so much more efficiently than ordinary heat engines can.
Conclusion: The law of entropy precludes all events which might lead to a decrease in entropy, even while obeying the energy law. Entropy thus reveals itself to be one of the most important and most remarkable concepts of physics.
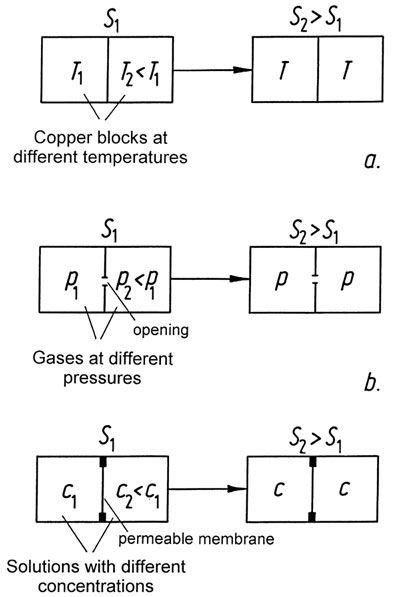 |
Figure 42: |
Entropy and disorder?
In countless publications, examples are given which illustrate that when the entropy of a system increases, the amount of disorder also increases; in other words, the orderliness is diminished. This idea has unfortunately also been extended to biological systems. The following arguments refute such a view:
– Biological processes take place in open systems, and are not closed. The second law allows a decrease in entropy as long as there is a corresponding increase in entropy in the environment. What is completely precluded is that the overall amount of entropy could be diminished.
– There can be no generally valid relationship between entropy and disorder, because entropy is a physical quantity which can be formulated exactly, but there is no exact formulation for disorder. The present author attempted a classification of the order concept in [G5], and different kinds of order are depicted in Figure 43.
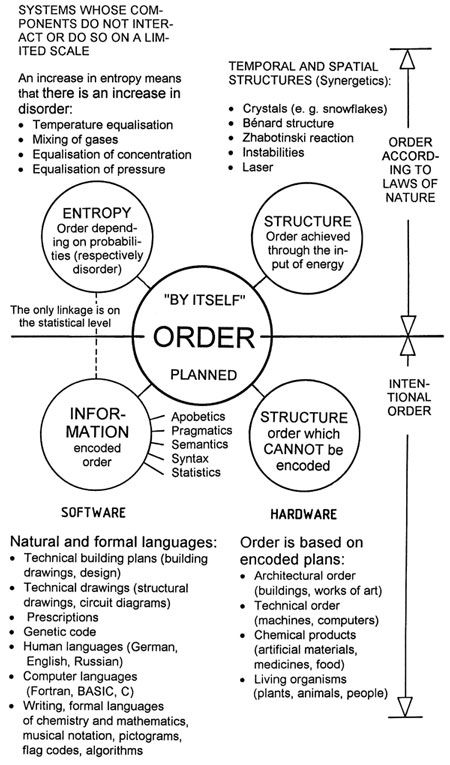 |
Figure 43: |
– The examples selected for illustrating the apparent relationship between entropy and disorder are, without exception, systems where there is no interaction between components. Such systems are irrelevant as far as biological structures are concerned, since thousands of chemical reactions take place within minute spaces.
– Biological order is based on the information present in all cells. The quality and quantity of this information should now be obvious — see chapter 6 and paragraph A1.2.3.
The ripple patterns produced in beach sand by retreating tides represent a certain order imparted by energetic processes (Figure 44), but there is no underlying code and also no intention. Such order does not represent information and thus cannot be stored.
 |
Figure 44: |
A3.2 Strategies for Maximizing the Utilization of Energy
The utilization and consumption of energy is always a question of converting one form of energy into another. When the energy produced by a certain source is utilized, the objective is to use the energy as economically as possible. In other words, maximum efficiency is the goal, employing a strategy for maximization. The following sections are devoted to a discussion of technical and biological systems where this principle is used. When energy is consumed, the inverse strategy becomes important, namely minimization of consumption: the available fuel must be used as economically as possible. The required work then has to be done with a minimal input of energy. The brilliant methods and the superlative results achieved by biological systems are discussed in paragraph A3.3.
A3.2.1 Utilization of Energy in Technological Systems
Man’s inventiveness has produced numerous concepts in the field of energy production and utilization. In most cases, the conversion of energy from the primary source to the consumer entails a number of forms of energy. For example, the chemical energy of fuel is converted into heat, the heat is then utilized to produce mechanical work, which, in its turn, is converted into electrical power. In a car engine, the chemical energy of the fuel changes into heat in the combustion chambers, and the explosive expansion of the gases is converted into mechanical work. An electric light bulb converts electrical energy into heat and light. Losses occur during all these conversions, and the ratio between the input energy and the recovered energy represents the efficiency of the process. Even in present-day coal-burning steam generating plants, the efficiency is only about 40%. This means that 60% of the chemical energy of the coal is lost.
