Life's Ratchet: How Molecular Machines Extract Order from Chaos (30 page)
Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann

A particularly intense debate is over the molecular motor myosin II. There are approximately eighteen different myosins in nature, and myosin II molecules make animal muscles work. When we decide to lift an arm, we send signals to countless myosin motors in our muscles and tell them to pull on actin fibers. Myosins grab the fibers and pull them taught, as in a game of tug-of-war. As the myosin motors pull in the fibers, our muscles contract and our arm lifts up. Next time you lift an arm (or move any part of your body), remember that this is accomplished by an army of myosin nanobots.
Measuring how these motors work at the single-molecule level is challenging, and unsurprisingly, different results have emerged from different laboratories. This has fueled the controversy. Until recently, most researchers believed that myosin II was a tightly coupled motor, making measured steps of five to ten nanometers per ATP molecule consumed. However, one of the pioneers of single molecular motors, Toshio Yanagida of Osaka University, produced repeated measurements that contradicted this claim. Yanagida claims that the distance myosin II moves fluctuates and can reach up to thirty nanometers per ATP molecule. This could only be possible if myosin were a loosely coupled motor.
Whichever way this controversy is resolved, the underlying debate over the relative role of the random thermal noise of the molecular storm is somewhat artificial. Even the most tightly coupled motor has to maneuver in the violent environment of the molecular storm. As we saw, a super-Hummer machine cannot work without occasionally loosening its grip. Once a molecular machine lets go, it is subjected to the random forces of thermal motion. A machine that can harness this random motion will be more efficient than a machine that cannot. As demonstrated, kinesin, a tightly coupled motor, uses the molecular storm to push its feet forward. The allosteric tilting of the molecule helps bias the movement in the forward direction, but where does the tilt come from? Any change in shape of a molecule is ultimately the result of the molecular storm’s pushing the molecule in the direction of reduced energy, that is, into a valley of its energy landscape. A molecular motor will simply not work if
the temperature is too low to provide sufficient random thermal motion (
Figure 6.12
). Even the most tightly controlled motor needs the chaos of the thermal dance to traverse transition states and find its way on an ever-changing energy landscape. Without the chaos of the molecular storm, the molecular motors in our cells would not move and we would be dead.
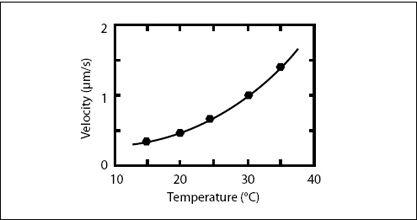
FIGURE 6.12.
Speed of a molecular motor as a function of temperature (reprinted from Kenji Kawaguchi and Shin’ichi Ishiwata, “Temperature Dependence of Force, Velocity, and Processivity of Single Kinesin Molecules,”
Biochemical and Biophysical Research Communications
272, no. 3 (2000): 895–899, with permission from Elsevier). The speed increases exponentially as temperature is increased—a behavior typical for chemical transformations that need thermal motion to overcome transition barriers.
Erwin Schrödinger quotation courtesy of Cambridge University Press. R. Dean Astumian epigraph quote courtesy of R. Dean Asumian; from R. Dean Asumian, “Making Molecules into Motors,”
Scientific American
(July 2001).
Twist and Route

Nature shows that molecules can serve as machines because living things work by means of such machinery. Enzymes are molecular machines that make, break, and rearrange the bonds holding other molecules together. Muscles are driven by molecular machines that haul fibers past one another. DNA serves as a data-storage system, transmitting digital instructions to molecular machines, the ribosomes, that manufacture protein molecules.
—K. E
RIC
D
REXLER
, “M
ACHINES OF
I
NNER
S
PACE
,”
IN
N
ANOTECHNOLOGY
: R
ESEARCH AND
P
ERSPECTIVES

T
HE CELL IS LIKE A CITY. THERE IS A LIBRARY (THE NUCLEUS, which contains the genetic material), power plants (mitochondria), highways (microtubules and actin filaments), trucks (kinesin and dynein), garbage disposals (lysosomes), city walls (membranes), post offices (Golgi apparatus), and many other structures fulfilling vital functions (
Figure 7.1
). All of these functions are performed by molecular machines. Some machines twist DNA; some route cargo along molecular highways or through the cell membrane. We now know that molecular machines are like tiny Maxwell’s demons—on a steady diet of ATP. We now have some inkling of how they work. But there are numerous such machines in living
cells, doing many different things, moving in different ways, working together in ways we still do not fully understand. In this chapter, we will look at some of these machines, find out what they do, why we need them, how they work, how scientists have unraveled their mysteries—and the questions that remain. We will find that life does not yield its secrets so easily.
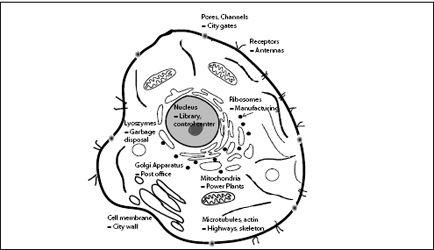
FIGURE 7.1.
Schematic of an animal cell.
In every city, there are menial jobs: lifting, carrying, rearranging, rebuilding. In the cell city, it’s no different. Most of these jobs are done by three types of molecular machines: kinesin, myosin, and dynein (
Figure 7.2
), which we will call molecular motors or motor proteins, from now on. There is not one type of kinesin, myosin, or dynein doing one type of job. Instead, like a fleet of customizable trucks, there are superfamilies of molecular motors, with eighteen known classes of myosins, ten classes of kinesins, and two classes of dyneins. Each class, in turn, has many members, resulting in a giant number of variations on a common theme. Humans have genes that encode something like 150 different kinesins, myosins, and dyneins, including 40 to 50 myosins alone. On the other hand, the humble
yeast fermenting your beer gets away with 5 myosins, which is still a lot for a single-celled organism.
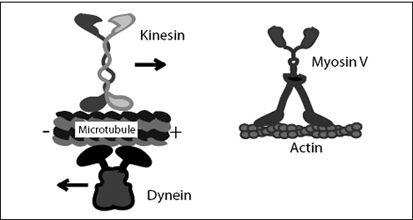
FIGURE 7.2.
Molecular transport motors in our cells. These walking molecules are about thirty to fifty nanometers in height.
The central part of each molecular motor is an ATPase. The suffix
-ase
signifies an enzyme, that is, a protein that facilitates a chemical reaction. In general, if the name of the enzyme is something like
X-ase
(pronounced “eks-ase”), the enzyme breaks down
X
. If the enzyme instead assists in the assembly of
X
, it is called an
X-synthase
. ATP synthase assembles ATP, while ATPase breaks down ATP. As discussed
Chapter 6
, the breakdown of ATP liberates a large amount of energy, which contributes most of the energy that drives a molecular motor. The ATPase site of the molecular motor is therefore the motor’s combustion chamber.
The combustion chamber, a molecular pocket, snugly fits one ATP molecule. This ATPase pocket facilitates
hydrolysis
, the breaking off of a phosphate group from ATP, which leaves behind ADP. Hydrolysis (the phosphate is removed when ATP interacts with a water molecule) liberates 5.8 × 10
−20
joules in energy, or 0.36 eV. This doesn’t sound like a lot, but it is actually a fair amount of energy—about fourteen times the average energy per molecule contained in the molecular storm. Such a high energy corresponds to locally heating a molecule to 3,900 degrees Celsius, or 7,000 degrees Fahrenheit. The hydrolysis of ATP also produces a small
change in the shape of the ATPase pocket, creating a displacement of about 0.5 nm. Knowing the energy and the displacement, we can calculate the maximum force that can be generated by the hydrolysis of ATP in the ATPase pocket: Assuming 100 percent efficiency, or assuming that all the released energy is available to do work, the force would equal the released energy divided by the displacement, or 5.8 × 10
−20
joules, divided by 0.5 × 10
−9
m (1 nm = 10
−9
m), which yields a force of 116 piconewtons (pN) (
pico
denotes one-trillionth, or 10
−12
). How big of a force is this? If we compare a force of 116 pN to the weight of the smallest visible dust particle, the weight of the dust particle is still larger by about a factor of 5. The energy released from one ATP molecule could lift the dust particle by a mere 0.1 nm, or the size of a hydrogen atom. This sounds incredibly tiny, but in the molecular world of our cells, 116 pN is a huge force. For example, it would be sufficient to rupture the membrane of the cell.
