Power, Sex, Suicide: Mitochondria and the Meaning of Life (18 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General

Something very similar happens in photosynthesis. In this case, the sun’s energy is used to pump protons across the chloroplast membrane in an analogous fashion to respiration. Bacteria, too, function in the same way as mitochondria, by generating a proton-motive force across their outer cell membrane. For anyone who is not a microbiologist, there is no field of biology more confusing than the astonishing versatility with which bacteria generate energy. They seem to be able to glean energy from virtually anything, from methane, to sulphur, to concrete. This extraordinary diversity is related at a deeper level. In each case, the principle is exactly the same: the electrons pass down a redox chain to a terminal electron acceptor (which may be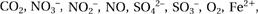 and others). In each case the energy derived from the redox reactions is used to pump protons across a membrane.
and others). In each case the energy derived from the redox reactions is used to pump protons across a membrane.
Such a deep unity is noteworthy not just for its universality, but perhaps even more because it is such a peculiar and roundabout way of generating energy. As Leslie Orgel put it, ‘Few would have laid money on cells generating energy with proton pumps.’ Yet proton pumping is the secret of photosynthesis, and all forms of respiration. In all of them, the energy released by redox reactions is used to pump protons across a membrane, to generate a proton-motive force. It seems that pumping protons across a membrane is as much a signature of life on earth as DNA. It is fundamental.
In fact the proton-motive force has a much broader significance than just generating ATP, as Mitchell realized. It acts as a kind of force field, enveloping bacteria with an impalpable source of power. Proton power is involved in several fundamental aspects of life, most notably the active transport of molecules in and out of the cell across the external membrane. Bacteria have dozens of membrane transporters, many of which use the proton-motive force to pump nutrients into the cell, or waste products out. Instead of using ATP to power active transport, bacteria use protons: they hive off a little energy from the proton gradient to power active transport. For example, the sugar lactose is transported into the cell against a concentration gradient by coupling its transport to the proton gradient: the membrane pump binds one lactose molecule and one proton, so the energetic cost of importing lactose is met by the dissipation of the proton gradient, not by ATP. Similarly, to maintain low sodium levels inside the cell, the removal of one sodium ion is paid for by the import of one proton, again dissipating the proton gradient without consuming ATP.
Sometimes the proton gradient is dissipated for its own sake, to produce heat. In these circumstances, respiration is said to be uncoupled, for electron flow and proton pumping continue as normal, but without ATP production. Instead, the protons pass back through pores in the membrane, thereby dissipating the energy bound up in the proton gradient as heat. This can be useful in itself, as a means of producing heat, as we shall see in
Part 4
, but it also helps to maintain electron flow during times of low demand, when ‘stagnant’ electrons are prone to escape from the respiratory chain to react with oxygen, producing destructive oxygen free radicals. Think of this like a hydroelectric dam on a river. At times of low demand there is a risk of flooding, which can be lowered by having an over-flow channel. Similarly, in the respiratory chain, a through-flow of electrons can be maintained by uncoupling electron flow from ATP synthesis. Instead of flowing through the main hydroelectric dam gates (the ATPase), some protons are diverted through the overflow channels (the membrane pores). This through-flow helps to prevent any problems that may arise from having an overflowing reservoir of electrons, ready to form free radicals; and there are important health consequences, as we shall see in later chapters.
Besides active transport, the proton force can be put to other forms of work. For example, bacterial locomotion also depends on the proton-motive force as shown by the American microbiologist Franklin Harold and his colleagues in the 1970s. Many bacteria move around by rotating a rigid corkscrew-like flagella attached to the cell surface. They can achieve speeds of up to several hundred cell-lengths per second by this process. The protein that rotates the flagellum is a tiny rotary motor, not dissimilar to the ATPase itself, which is powered by the proton current through a drive shaft.
In short, bacteria are basically proton-powered. Even though ATP is said to
be the universal energy currency, it isn’t used for all aspects of the cell. Both bacterial homeostasis (the active transport of molecules in and out of the cell) and locomotion (flagellar propulsion) depend on proton power rather than ATP. Taken together, these vital uses of the proton gradient explain why the respiratory chain pumps more protons than are required for ATP synthesis alone, and why it is hard to specify the number of ATP molecules that are formed from the passage of one electron—the proton gradient is fundamental to many aspects of life besides ATP formation, all of which tap off a little.
The importance of the proton gradient also explains the odd propensity of the ATPase to go into reverse, pumping protons at the cost of burning up ATP. On the face of it, such a reversal of the ATPase might seem to be a liability, because it swiftly drains the cell of its ATP reserves. This only begins to make sense when we appreciate that the proton gradient is more important than ATP. Bacteria need a fully charged proton-motive force to survive, just as much as a galactic cruiser in Star Wars needs its protective force field fully operational before attacking the Empire’s star fleet. The proton-motive force is usually charged up by respiration. However, if respiration fails, then bacteria generate ATP by fermentation. Now everything goes into reverse. The ATPase immediately breaks down the freshly made ATP and uses the energy released to pump protons across the membrane, maintaining the charge—which amounts to an emergency repair of the force field. All other ATP-dependent tasks, even those as essential as DNA replication and reproduction, must wait. In these circumstances, it might be said that the main purpose of fermentation is to maintain the proton-motive force. It is more important for a cell to maintain its proton charge than it is to have an ATP pool available for other critical tasks such as reproduction.
To me, all this hints at the deep antiquity of proton pumping. It is the first and foremost need of the bacterial cell, its life-support machine. It is a deeply unifying mechanism, common to all three domains of life, and central to all forms of respiration, to photosynthesis, and to other aspects of bacterial life, including homeostasis and locomotion. It is in short a fundamental property of life. And in line with this idea, there are good reasons to think that the origin of life itself was tied to the natural energy of proton gradients.
The Origin of Life
How life began on Earth is one of the most exhilarating fields of science today—a wild west of ideas, theories, speculations, and even data. It is too large a subject to embark on in detail here, so I will limit myself to a few observations on the importance of chemiosmotics. But for perspective let me paint a quick picture of the problem.
The evolution of life depends in very large measure on the power of natural selection—and this in turn depends on the inheritance of characteristics that can be subjected to natural selection. Today we inherit genes made of DNA; but DNA is a complicated molecule and can’t have just ‘popped’ into existence. Moreover, DNA is chemically inert, as we noted in the Introduction. Recall that DNA does little more than code for proteins, and even this is achieved by way of a more active intermediary, RNA, which in various forms physically translates the DNA code into the sequence of amino acids in a protein. In general, proteins are the active ingredients that make life possible—they alone have the versatility of structure and function needed to fulfil the multifarious requirements of even the simplest forms of life. Individual proteins are honed to the requirements of their particular tasks by natural selection. First among these tasks, proteins are needed to replicate DNA and to form RNA from the DNA template, for without heredity natural selection is not possible; and for all their glories proteins are not repetitive enough in structure to form a good heritable code. The origin of the genetic code is therefore a chicken and egg problem. Proteins need DNA to evolve, but DNA needs proteins to evolve. How did it all get started?
The answer agreed by most of the field today is that the intermediary, RNA, used to be central. RNA is simpler than DNA, and can even be put together in a test tube by chemists, so we can bring ourselves to believe that it may once have formed spontaneously on the early Earth or in space. Plenty of organic molecules, including some of the building blocks of RNA, have been found on comets. RNA can replicate itself in a similar manner to DNA, and so forms a replicating unit that natural selection can act upon. It can also code for proteins directly, as indeed it does today, and so provides a link between template and function. Unlike DNA, RNA is not chemically inert—it folds into complex
shapes and is able to catalyse some chemical reactions in the same way as enzymes (RNA catalysts are called ribozymes). Thus, researchers into the origin of life point to a primordial ‘RNA world’, in which natural selection acts upon independently self-replicating RNA molecules, which slowly accrue complexity, until being displaced by the more robust and efficient combination of DNA and proteins. If this whistlestop tour whets your appetite for more, I can recommend
Life Evolving
by Christian de Duve as a good place to start.
Elegant as it is, there are two serious problems with the ‘RNA world’. First, ribozymes are not very versatile catalysts, and even allowing for the most rudimentary catalytic efficiency, there is a big question mark over whether they could have brought a complex world into existence. To me they are rather less suitable than minerals as the original catalysts. Metals and minerals are found at the heart of many enzymes today, including iron, sulphur, manganese, copper, magnesium, and zinc. In all these cases, the enzyme reaction is catalysed by the mineral (technically, the prosthetic group), not the protein, which improves the efficiency rather than the nature of the reaction.
Second, more importantly, there is an accounting problem with energy and thermodynamics. The replication of RNA is work, and therefore requires an input of energy. The requirement for energy is constant, because RNA is not very stable, and is easily broken down. Where did this energy come from? There were plenty of sources of energy on the early earth, mooted by astrobiologists—the impact of meteorites, electrical storms, the intense heat of volcanic eruptions, or underwater hydrothermal vents, to name but a few. But how these diverse forms of energy were converted into something that life could use is rarely described—none of them is used directly, even today. Probably the most sensible suggestion, which has been in and out of favour over several decades, is the fermentation of a ‘primordial soup’, cooked up by a combination of all the various forms of energy.
The idea of a primordial soup gained experimental support in the 1950s, when Stanley Miller and Harold Urey passed electric sparks, to simulate lightning, through a mixture of gases believed to represent the earth’s early atmosphere—hydrogen, methane, and ammonia. They succeeded in producing a rich mixture of organic molecules, including some precursors of life, such as amino acids. Their ideas fell out of favour because there is no evidence that the earth’s atmosphere ever contained these gases in sufficient quantities; and organic molecules are far harder to form in the more oxidizing atmosphere now thought to have existed. But the existence of plentiful organic material on comets has brought us round full circle. Many astrobiologists, keen to link life with space, argue that the primordial soup could have been cooked up in outer space. The earth then received generous helpings in the huge asteroid bombardment that pockmarked the moon and earth for half a billion years from 4.5
to 4 billion years ago. If the soup really did exist, then perhaps life could have started out by fermenting a soup after all.
But there are several problems with fermentation as the original source of energy. First, as we have seen, fermentation stands apart from both respiration and photosynthesis, in that it does not pump protons across a membrane. This leads to a discontinuity and a problem with time. If all the fermentable organic compounds came from outer space, then the nutrient supply should have begun to run out after the great asteroid bombardment drew to a close 4 billion years ago. Life would dribble away to extinction unless it could invent photosynthesis, or some other way of producing organic molecules from the elements, before the fermentable substrates ran out. And this is where we run into a problem with time. Traces of fossil evidence suggest that life on Earth began at least 3.85 billion years ago, and that photosynthesis evolved some time between 3.5 billion and 2.7 billion years ago (although this evidence has been questioned lately). Given the discontinuity between fermentation and photosynthesis—not a single intermediary step brings us any loser to the evolution of photosynthesis—then the gap of at least several hundred million years, and perhaps a billion years, looks very awkward. With no other source of energy, could the organic molecules delivered by asteroids really nourish life for that long? It doesn’t sound very likely to me, especially given the tendency of ultraviolet radiation to break down complex organic molecules in the days before the ozone layer.
