Power Up Your Brain (19 page)
Read Power Up Your Brain Online
Authors: David Perlmutter M. D.,Alberto Villoldo Ph.d.
Tags: #Health & Fitness, #General, #ebook, #book

However, free radicals cause oxidative damage to tissues, proteins, fat, and even nuclear DNA. In fact, tissue damage by free radicals is thought to underlie the process of aging. As we saw in Chapter 4, Denham Harman laid the groundwork for the antioxidant industry when he demonstrated that antioxidants “quench” free radicals in 1956. Then, in 1972, Harman recognized that mitochondria, which, ironically, are the actual source of free radicals, are also most at risk of damage from free radicals. Because the brain produces a prodigious amount of free radicals, it is their primary target, yet the brain lacks the level of antioxidant protection generated by other cells elsewhere in the body.
FREE RADICALS
Because of the powerfully damaging effects of free radicals, especially in regard to the brain, researchers are seeking better antioxidants that will provide brain cells with a measure of protection to stave off mitochondrial breakdown and, perhaps, enhance brain function as well. Studies are now appearing that clearly point an accusatory finger at free radicals for playing a pivotal role in brain aging. These studies show that, essentially, when a person begins to have too many “senior moments,” clinicians apply a more scientific term,
mild cognitive impairment
(MCI). This phenomenon is generating considerable interest because MCI generally presages a more sinister pathology, Alzheimer’s disease.
The relationship between MCI and free radicals was well described in a 2007 report by William Markesbery, a neurologist at the University of Kentucky, which demonstrated that cognitive function begins to decline well before the Alzheimer’s stage and that the greater the oxidative damage to fat, protein, and even nuclear DNA, the greater the degree of mental impairment. Markesbery clearly identifies oxidative damage as a “therapeutic target to slow the progression or perhaps the onset of the disease.”
1
What a concept: to therapeutically target free radicals in an attempt to prevent Alzheimer’s! What a refreshing approach published by the American Medical Association. Rather than simply describe some new drug therapy for a disease that’s already well under way, here is a preventive medicine model, applied to brain health.
Markesbery goes on to state, “Better antioxidants and agents used in combination to upregulate defense mechanisms against oxidation will be required to neutralize the oxidative component of the pathogenesis of Alzheimer’s disease. It is most likely that to optimize these neuroprotective agents, they will have to be used in the presymptomatic phase of the disease.”
2
That last phrase means during the time of mild cognitive impairment or even
before
the appearance of symptoms. In other words,
you are never too young
to begin saving your mind for a healthier, longer “old age.” And when we recognize that the risk of contracting Alzheimer’s by living to be 85 years or longer is an astounding 50 percent, there are a lot of people who would be wise to consider that they are “presymptomatic” right now.
ORAL ANTIOXIDANTS
So, if in fact our brain tissue is being assaulted by free radicals, does it make sense to load up with antioxidants? To answer the question, let’s go back to the mitochondria. In the normal process of producing energy, each mitochondrion produces hundreds, if not thousands, of free radical molecules each day. Multiple that by the ten million billion mitochondria in your brain and you come up with an unfathomable number—10 followed by 18 zeroes. So, you might ask: Just how effective is a vitamin E capsule or a tablet of vitamin C when confronted by this onslaught of free radicals? Are one or two little pills once or twice a day up to the task?
When confronted by a free radical, an antioxidant sacrifices itself to oxidation in a one-to-one reaction. Thus, one molecule of vitamin C becomes oxidized by one free radical. Yes, this neutralizes the free radical, but it also terminates the vitamin C molecule. Can you imagine how much vitamin C or other oral antioxidant you would need to take in order to neutralize the astronomical number of ROS molecules generated by the body on a daily basis?
As you might expect, human physiology has developed its own biochemistry to deal with the free radical fire. Far from being entirely dependent on antioxidants from externally derived food sources, your cells have their own innate ability to generate antioxidant enzymes upon demand when environmental signals to the cell tell the nuclear DNA to do so. Fortunately, this innate and internal antioxidant system is far more powerful than any nutritional supplement. Whether the juice of some exotic berry or an extract from a previously unknown jungle plant, antioxidant supplements are limited by stoichiometric chemistry. The golden key to antioxidant protection lies in your nuclear DNA. Now let’s learn how to activate the switch.
NRF2 PROTEIN
Nrf2 Protein and Antioxidants
When the body experiences high oxidative stress and produces an excess number of free radicals, it also activates a specific protein in the nucleus called Nrf2. This is a very important protein, because it opens the door for production of a vast array of your body’s most important antioxidants as well as detoxification enzymes.
But what activates Nrf2?
This is where the story gets really exciting because the answer is: a variety of modifiable factors.
Vanderbilt University’s Dr. Ling Gao has found that oxidation of the omega-3 fats eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) activates the Nrf2 pathway in dramatic fashion. For years, researchers have noted decreased levels of free radical damage in individuals who consumed fish oils, the source of EPA and DHA, but this new research clarifies the relationship between fish oil and antioxidant protection. As Dr. Gao reported, “Our data support the hypothesis that the formation of . . . compounds generated from oxidation of EPA and DHA in vivo can reach concentrations high enough to induce Nrf2-based antioxidant and . . . detoxification defense systems.”
3
Calorie reduction, as demonstrated in a variety of laboratory models, also induces Nrf2 activation. When calories are reduced in the diets of laboratory animals, not only do they live longer, likely as a result of increased antioxidant protection, but they become remarkably resistant to a variety of forms of cancer. This attribute of Nrf2 further supports the fasting program that you will learn about in Chapter 14, “The Power Up Your Brain Program.”
Over the past several years, Nrf2 chemistry has become a global focal point for medical research. This has led to the discovery that a variety of natural compounds activates and amplifies the genes responsible for the production of a vast complex of protective and life-sustaining detoxification enzymes and antioxidants. Among these are curcumin, which comes from turmeric; green tea extract; resveratrol; sulphoraphane, derived from broccoli; and the omega-3 fat, DHA. In activating the Nrf2 pathway, these natural substances enhance the body’s production of glutathione, what may be the most important brain antioxidant in human physiology.
So powerful is the antioxidant protection offered by Nrf2-induced glutathione that it was able to prevent amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease) in the laboratory animal model of this disease.
4
Nrf2 Protein and Inflammation
In addition to its antioxidant functionality, activation of the Nrf2 pathway turns on the genes that produce a vast array of protective chemicals in two other critically important areas: inflammation reduction and detoxification, which are also subjects of this chapter.
At first blush, the subject of inflammation might seem out of place in a discussion regarding enhanced brain health and functionality. But, while we are all familiar with inflammation as it relates to such disease states as arthritis and asthma, the past decade has produced an extensive body of research that connects inflammation with a variety of neurodegenerative conditions. Indeed, research clearly demonstrates a remarkable reduction in incidence of both Parkinson’s and Alzheimer’s in individuals who have taken NSAIDs for a number of years.
5
Other studies also show dramatic elevation of cytokines, which are the cellular mediators of inflammation, in the brains of individuals with these and other degenerative brain disorders.
New technology now allows MRI and PET scan imaging of cells that actively produce inflammatory cytokines in the brains of Alzheimer’s patients.
6
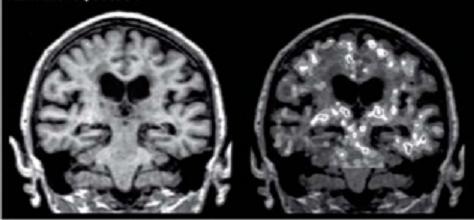
With this knowledge, we are now forced to regard inflammation in a whole new light. Far more than just the cause of your painful knee or sprained ankle, inflammation underpins the very process of brain degeneration. Ultimately, the key downstream effect of inflammation in the brain is that it is responsible for the damage that prevents activation of Nrf2 chemical pathways and the consequential increase of free radical production. On the positive side, turning on the Nrf2 pathway not only reduces free radicals directly but, as a bonus, reduces inflammation, which in turn protects the brain from excess free radicals as a result of inflammation. Do you see a positive cycle here?
Interventions designed to reduce inflammation through the use of natural substances, such as turmeric, have been described in medical literature for more than 2,000 years. But only in the past decade have we begun to understand the intricate and eloquent biochemistry that explains what traditional health-care practitioners have known and utilized for millennia. Indeed, food choices have controlled humanity’s DNA expression for as long as our species has walked the planet.
Nrf2 Protein and Detoxification
The third and no less important benefit of the Nrf2 pathway is that it activates specific genes that produce enzymes and other chemicals that break down and eliminate toxins. You might wonder why your DNA would contain codes for the production of detoxification chemicals. After all, didn’t humanity’s first real exposure to toxins begin late in the history of humanity, with the industrial era? Well, no.
Some of the most dangerous toxins, such as lead, arsenic, and aluminum, exist
naturally
in the environment. Plants and animals also generate powerful toxins as a form of protection. Our human bodies also produce toxins during metabolism; even the carbon dioxide that we exhale is a poison to our systems, but, fortunately, it is essential for plants, which convert it, through photosynthesis, back into oxygen that we can breathe.
For these reasons, our detoxification system has served us for a very long time. Likewise, today, we are just beginning to understand how natural substances, such as turmeric, have also served as detoxification agents through their ability to enhance genetic expression. In fact, turmeric’s ability to activate detoxification genes explains why it’s also able to completely eliminate the damaging effects of radiation chemotherapies in laboratory animals.
7
The human body produces an impressive array of enzymes that detoxify poisons to which we are exposed both externally and internally. Our DNA produces these detoxification enzymes, which have evolved over hundreds of thousands of years in response to our intrinsic requirements and as protection mechanisms for our ancestors as they migrated to new surroundings. For millennia, these internal defense mechanisms evolved, for the most part, somewhat slowly. However, over the past century, human physiology has been challenged by an incomprehensible array of novel chemical toxins for which our genetic detoxification endowment was unprepared.
It is as if we are functioning with outdated machinery, hoping against all odds that, somehow, our physiology will be able to deal with an unprecedented onslaught of toxins. And we are asking a lot of our body. But the good news is that we are endowed with a detoxification system with far-reaching potential. This is an important consideration because so many of the toxins to which we are exposed every single day are directly toxic to the brain.
GLUTATHIONE AND DETOXIFICATION
A significant player in detoxification chemistry is glutathione. It binds to various toxins and renders them less noxious. Most important, glutathione is a substrate for the enzyme glutathione S-transferase, which helps transform a multitude of toxins into forms that are more water soluble and thus more easily excreted.
