The Cerebellum: Brain for an Implicit Self (15 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

Conjunctive LTD is a unique phenomenon at parallel fiber-Purkinje cell synapses: for example, it does not occur in synapses formed by ascending granule cell axons and Purkinje cell dendrites (
Sims and Hartell, 2006
). Furthermore, conjunctive LTD is input-specific. It occurs only in parallel fiber-Purkinje cell synapses involved in conjunctive stimulation (
Ito et al., 1982
;
Ekerot and Kano, 1985
). This input specificity is maintained in cultured Purkinje cells, in which LTD-like depression occurs only on the part of the dendrite exposed to quisqualate, but not in other unexposed parts (
Linden, 1994
). Nevertheless, in rat cerebellar slices, Wang et al. (
2000b
) estimated that conjunctive LTD spreads from a small number (~5-30) of stimulated parallel fiber synapses to ~20,000 unstimulated parallel fiber synapses. Thus, LTD must spread beyond conjunctively activated parallel fibers to about 600-fold (20,000/30) more parallel fiber synapses in the immediate neighborhood. The magnitude of this spread decreases to ~50% of the maximum 50 micrometers away from the conjunctively activated site. Such a spread, however, occurs only in neighboring synapses that are active at a very low rate of discharge (~0.2 Hz) and it has only a loose temporal relationship with climbing fiber activity (
Reynolds and Hartell, 2000
). Thus, the principle of input specificity for conjunctive LTD still holds in a broad sense.
In attempts to analyze the mechanisms of conjunctive LTD, reduced forms of its manifestation are often examined. These have been induced by replacing parallel fiber- or climbing fiber stimulation with various pharmacological reagents. For example, iontophoresis of glutamate or quisqualate onto Purkinje cells, but not kainate and aspartate, has been used to replace parallel fiber stimulation (
Ito et al., 1982
;
Kano and Kato, 1987
). In another study, strong depolarizing pulses, which induced the entry of Ca
2+
ions into Purkinje cells through voltage-gated channels, were used to replace climbing fiber stimulation (
Crepel and Kruppa, 1988
;
Crepel and Jaillard, 1991
).
Other simple procedures for inducing LTD were to apply quisqualate (
Yuzaki et al., 1994
) that stimulates both AMPA receptors and metabotropic glutamate receptor type 1 (mGluR1) receptors, or to increase extracellular K
+
concentration (
Crepel et al., 1994
). Intracellular photo-release of free Ca
2+
ions from “caged” Ca
2+
ions induced LTD effectively (
Finch and Augustine, 1998
). Also, Linden and his colleagues used to good effect glutamate/depolarization conjunction to induce LTD in cultured cells (
Linden et al., 1991
).
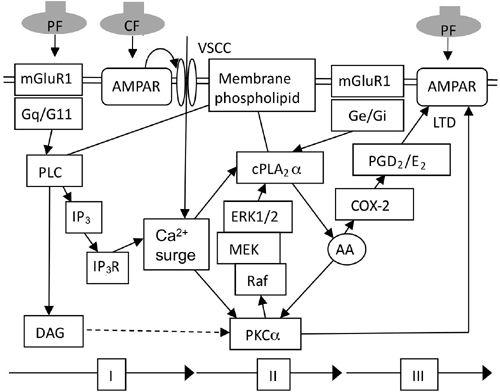
Complex signal transduction underlying synaptic plasticity in cerebellar neurons has been analyzed extensively and in detail, particularly with respect to conjunctive LTD (
Figure 19
). Among the many molecules involved, some are indispensable for LTD induction, but others are required only under selected experimental conditions. According to the convention for LTP (
Sanes and Lichtman 1999
), such molecules will be defined as mediators and modulators, respectively (
Ito, 2002
).
Figure 19. Signal transduction underlying LTD.
Chain of reactions induced by conjunctive stimulation of parallel fibers (PF) and climbing fibers (CF). I, II, and III signify the three phases of the signal transduction process. Additional abbreviations: AA, arachidonic acid; COX-2, cyclooxygenase type 2; cPLA
2
α; cytosolic phospholipase A 2 alpha subtype; DAG, diacylglycerol; ERK, extracellular signal-regulated kinase (belong to MAP kinases); G11/Ge/Gi/Gq, subtypes of G proteins; IP
3
, inositol 1,4,5-trisphosphate; IP
3
R, IP
3
receptor; MEK, MAPK/ERK kinase; mGluR1, metabotropic glutamate receptor type 1; PGD
2
/E
2
, prostaglandin D
2
and E
2
; PLC, phospholipase C (PLCβ3 or 4 subtypes); Raf, kinase related to MAP kinase; VSCC, voltage-sensitive Ca
2+
channel.
Ca
2+
surge in Purkinje cell dendrites.
The first step in the induction of conjunctive LTD can occur in two ways. One is by Ca
2+
entry through the voltage-gated Ca
2+
channels, which are called “P/Q” channels. The P channel was discovered in cerebellar Purkinje cells and named P in keeping with the first letter in Purkinje (Llinás and Sugimori, 1980;
Llinás et al., 1989
). The Q channel is a phenotypic variant of a P channel that is generated by gene splicing (
Bourinet et al., 1999
). P/Q channels are distributed throughout Purkinje cell somata and their entire dendritic trees (
Westenbroek et al., 1995
). Ca
2+
entry occurs during membrane depolarization caused by either climbing fiber responses or by parallel-fiber-evoked EPSPs. The other way is for Ca
2+
to be released from intracellular Ca
2+
stored in the endoplasmic reticulum. This release is triggered by the following steps. Activation of mGluR1 activates, in turn, the G-protein (Gq/11) (
Mailleux et al., 1992
;
Sternweis and Smrcka, 1993
;
Tanaka et al., 2000
), which, again in turn, activates certain subtypes of phospholipase C (PLCβ3 or 4) (
Roustan et al., 1995
;
Watanabe et al., 1998
;
Sugiyama et al., 1999
). The latter then hydrolyzes the membrane’s phosphatidyl-inositol 4,5-diphosphate (PIP
2
) to produce two second-messengers; inositol-trisphosphate (IP
3
) and 1,2 diacylglycerol (DAG). Finally, IP
3
acts on IP
3
receptors located on the membrane of the endoplasmic reticulum and induces Ca
2+
release. In addition, Ca
2+
also affects the gating of the IP
3
receptor channel as a co-agonist of the IP
3
receptor, together with IP
3
(
Iino, 1990
;
Bezprozvanny et al., 1991
;
Finch et al., 1991
). A kinetic model of Ca
2+
dynamics within a Purkinje dendritic spine was formulated assuming that (1) IP
3
was generated
slowly via the metabotropic pathway of parallel fiber inputs; and (2) the climbing fiber-evoked Ca
2+
influx triggered regenerative Ca
2+
-induced Ca
2+
release from the internal stores. The delay in IP
3
increase caused by the slow parallel fiber metabotropic pathway explained the optimal parallel fiber-climbing fiber stimulation interval (50–200 ms) for inducing LTD (
Doi et al., 2005
).
Importantly, the coincident activation of parallel fibers and a climbing fiber converging onto the test Purkinje cell produces Ca
2+
signals that are much larger than the linear sum of responses to either parallel fiber- or climbing fiber activation alone: that is, ~8 times in spines and ~4.5 times in dendritic shafts (
Wang et al., 2000a
).
This supralinear summation of climbing-fiber-evoked Ca
2+
entry and parallel-fiber-evoked release of Ca
2+
leads to an abrupt surge in the Ca
2+
concentration of Purkinje cell dendrites, as shown in Color Plate XII. Note in curves b–d in C that the Ca
2+
indicator fluorescence sharply increases to a peak and then decreases to the baseline at each successive conjunctive pulse.
The requirement of mGluR1-initiated signal transduction for the induction of conjunctive LTD has been shown by various means to interfere with signal transduction. These include (1) a specific antagonist of mGluR1, (RS)-α-methyl-4-carboxyphenylglycine (MCPG) (
Hartell, 1994
), (2) antibodies inactivating mGluR1 (
Shigemoto et al., 1994
), and (3) mGluR1-deficiency gene knockout (
Conquet et al., 1994
). In mice deficient in Gaq, conjunctive LTD was shown to be lacking (
Miyata et al., 1998
), albeit with the reservation that this mutant has climbing fibers that multiply innervate a single Purkinje cell (
Offermanns et al., 1997
). This aberrant innervation might impair the production of conjunctive LTD. On the other hand, the activation of mGluRs by an agonist (e.g., trans-ACPD or 1S, 3R-ACPD) has been shown to induce LTD when combined with depolarization-evoked Ca
2+
spikes. This was shown to occur even in the presence of CNQX, an antagonist of AMPA receptors (
Daniel et al., 1993
;
Hemart et al., 1995
). In another study a PLC inhibitor greatly attenuated an increase in mGluR-agonist-induced intracellular Ca
2+
concentration (
Netzeband et al., 1997
). The parallel-fiber-stimulation-evoked Ca
2+
increase was also shown to be blocked in mice deficient in the PLCβ4 gene (
Miyata et al., 2001
).
The importance of IP
3
-induced Ca
2+
release for induction of LTD has been shown by the following observations. (1) Application of heparin, a nonspecific inhibitor of the IP
3
receptor, blocked LTD induced by either glutamate/depolarization conjunction in cultured Purkinje cells (
Kasono and Hirano, 1995
) or parallel fiber/depolarization conjunction in Purkinje cells in a cerebellar slice (
Khodakhah and Armstrong, 1997
). (2) A specific antibody against the IP
3
receptor blocked LTD induction, and mice with a disrupted IP
3
receptor type 1 gene completely lacked LTD (
Inoue et al., 1998
). (3) Both LTD and IP
3
-mediated Ca
2+
signaling in dendritic spines of Purkinje cells were absent in mice and rats with mutations in myosin-Va that prevented endoplasmic reticulum from entering the cell’s dendritic spines. This loss of LTD was rescued by photolysis of a caged Ca
2+
compound (
Miyata et al., 2000
). (4) The photolytic release of caged IP
3
in peripheral dendrites of a Purkinje cell produced a strong and persistent Ca
2+
signal and evoked persistent LTD-like depression (
Finch and Augustine, 1998
).
On the other hand, in cultured or freshly isolated Purkinje cells, IP
3
was not required for inducing LTD by glutamate/depolarization conjunction. A potent and selective IP
3
receptor channel blocker, xestospongin C, did not affect this LTD
induction (
Narashimhan et al., 1998
). IP
3
is therefore not required for LTD induction in cultured or freshly isolated Purkinje cells, and hence IP
3
is a modulator, not mediator, for LTD induction.
PKCα and lipid-signaling cascade.
In Purkinje cell dendrites, a conjunction-evoked surge in Ca
2+
ions should activate two Ca
2+
-sensitive enzymes: PKCα and cPLA
2
α. DAG generated by PLC from membrane PIP2, in parallel with the production of IP
3
(see above), should also contribute to the activation of PKCα. The activated PKCα induces the phosphorylation of AMPA receptors in parallel fiber-Purkinje cell synapses. On the other hand, the activated cPLA
2
α initiates a positive feedback cycle consisting of (1) cPLA
2
α releasing arachidonic acid from membrane phospholipids, (2) arachidonic acid activating PKCα, (3) PKCα activating sequentially a series of kinases, Raf, MEK, and ERK1/2, and (4) these kinases then activating cPLA
2
α (
Figure 19
).
This cyclic activation involving PKCα and cPLA
2
α has been postulated to strengthen the activation of PKCα (
Kuroda et al., 2001
;
Tanaka and Augustine, 2008
). However, it has been shown quite recently that arachidonic acid also acts on conjunctive LTD via a cascade involving cyclooxygenase-2 (COX-2) and prostaglandin D
2
or E
2
(PGD
2
/PGE
2
) (
Le et al., 2010
) (
Figure 19
). Because either a PKC inhibitor or a COX-2 inhibitor blocks LTD, it appears that PKCα and PGD2/E2 act synergistically on AMPA receptors to dissociate them from the cytoskeleton (see below).
Events in AMPA receptors.
AMPA receptors are the sole ionotropic glutamate receptors in parallel fiber-Purkinje cell synapses; there are no NMDA receptors except during an early postnatal period. In conjunctive LTD induction, AMPA receptors are phosphorylated at serine-880 of the GluR2 COOH terminus and become dissociated from the subsynaptic cytoskeleton (
Matsuda et al., 2000
;
Xia et al., 2000
). The dissociated AMPA receptors are eventually eliminated from dendritic spines by endocytosis (for review, see
Carroll et al., 2001
). Such shift of AMPA receptors from synaptic membrane to inside of dendritic membrane has been demonstrated by immunolabeling AMPA recptors with an antibody binding the extracellular domain of GluR2/3 (Color Plates XIII and XIV). The size of parallel fiber-Purkinje cell EPSCs reflects the number of AMPA receptor molecules present in the synaptic membrane (
Linden, 2001
). The number of AMPA receptors is maintained by the balance between endocytotic elimination and exocytotic insertion. To explain AMPA receptor behavior during conjunctive LTD, their stable and mobile pools in the synaptic membrane and their internal mobile pool are in dynamic equilibrium within each dendritic spine. It is assumed currently that during conjunction, a portion of the stable synaptic pool of AMPA receptors is
shifted to the mobile synaptic pool and then moved to the internal mobile pool as schematically illustrated in (
Figure 20
).
