The Life of Super-Earths (11 page)
Read The Life of Super-Earths Online
Authors: Dimitar Sasselov

Smaller thingsâour bodies, for exampleâare held likewise together in a pressure balance, but it is not the balance between gravity and electromagnetic forces. (Our bodies have
too little mass for our own gravity to help hold us together.) Instead, we are pulled down by the gravity of planet Earth and simultaneously we are under pressure by the air above our headsâabout fifteen pounds of it on each square inch. The air, of course, is also pulled down by gravity. As we go to even smaller scales, electromagnetism gets more and more dominant and gravity all but drops out of the competition. Electromagnetism, as manifested by the chemical bond, makes our tissues structurally sound, keeping cells and larger multicell bodies together.
too little mass for our own gravity to help hold us together.) Instead, we are pulled down by the gravity of planet Earth and simultaneously we are under pressure by the air above our headsâabout fifteen pounds of it on each square inch. The air, of course, is also pulled down by gravity. As we go to even smaller scales, electromagnetism gets more and more dominant and gravity all but drops out of the competition. Electromagnetism, as manifested by the chemical bond, makes our tissues structurally sound, keeping cells and larger multicell bodies together.
Â
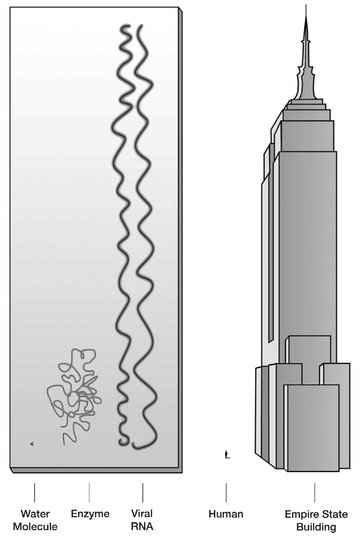
FIGURE 7.1
.
Comparative sizes: the molecules of life are huge when compared to the common molecule of water (H
2
O); they define the large-molecule scale.
.
Comparative sizes: the molecules of life are huge when compared to the common molecule of water (H
2
O); they define the large-molecule scale.
To everything, then, it seems there is the right size, as J. B. S. Haldaneâan early geneticist and famous science popularizerâmused in a 1928 essay. He was answering the question we always ask as children: How are insects built to be able to remain unscathed after a fall from a height that is multiple times their size? Why do some insects walk on water while others drown in it?
Haldane gave the right answers, but only in a general sense. A former colleague, Bill Press, was more mathematical about it and got the correct answer for the size of the human body. To get the correct answer, he had to make three assumptions: that a human body (1) is made of complicated molecules that are strings (polymers) rather than amorphous, (2) requires an atmosphere that is not of hydrogen and helium, and (3) is as large as possible to carry its large brain, but liable to stumble and fall, and in so doing should not break.
5
Thus he defined the right size of an active animal living on the solid surface of planet Earth. That includes the ambient temperature at which
humans (and most animals) live, whichânot surprisinglyâis very close to the temperature of their chemical bond energy. As Bill points out, that fact makes cooking practicable.
5
Thus he defined the right size of an active animal living on the solid surface of planet Earth. That includes the ambient temperature at which
humans (and most animals) live, whichânot surprisinglyâis very close to the temperature of their chemical bond energy. As Bill points out, that fact makes cooking practicable.
The big Universe, however, is definitely not built to our human scale, and the problem is not only the vastness of space, but also the unimaginable scale of time. Time and space are inseparably linked, as we learned from twentieth-century physics, so it should not be surprising that in a big place things take a long time to change. It is essential to my story that you get a feel for this. Nevertheless, while the Universe as a whole is not built to a human scale, it is obviously possible to find places that are, for example, our home planet. This, as I will argue in the next chapter, is not an accident. Life needs planets. Let's see why.
CHAPTER EIGHT
ORIGINS OF LIFE
Why Planets?
Â
Â
Â
Â
D
uring the Middle Ages, when every continent was a separate world, people seriously considered multiple origins.
1
During the Renaissance, with Earth now just another planet, people seriously considered multiple origins of life on the other planets, as well as the Moon. Thus I am bringing up an old question and revisiting it with fresh evidence about newly discovered worlds and about what it takes for life to emerge and survive on them. Some of this evidence I have touched on earlier, but I am going to bring together the various threads here to argue that for life to emerge, it must happen on planets.
uring the Middle Ages, when every continent was a separate world, people seriously considered multiple origins.
1
During the Renaissance, with Earth now just another planet, people seriously considered multiple origins of life on the other planets, as well as the Moon. Thus I am bringing up an old question and revisiting it with fresh evidence about newly discovered worlds and about what it takes for life to emerge and survive on them. Some of this evidence I have touched on earlier, but I am going to bring together the various threads here to argue that for life to emerge, it must happen on planets.
So, let's take a step back and think of the cosmic perspective. Why planets? Aren't there other places in the Universe that could be equally good cradles for life?
Here is a recipe to answer these questions: get what we know about other places in the Universe and mix it with what we know about life, and see if anything useful comes out. The first ingredient is easy to procure. During the past fifty years astronomers have revolutionized our knowledge of the observable Universe and have a pretty complete census of what is in itâlots of galaxies, many more stars, and a list of planets that grows practically by the day.
What we know about life is the difficult partâwe know a lot and very little, at the same time. Scientists have revolutionized our understanding of the building blocks and the amazing interconnectedness of living forms, yet the parts are in a system,
e
they form networks,
f
and there is something essential but elusive in all that.
e
they form networks,
f
and there is something essential but elusive in all that.
I'll begin with the difficult part and list what we know about lifeânot a definition of life, but just some essential attributes:
2
2
1. Life is chemical in essence.
3
3
2. Life is a system that is not in equilibrium.
4
4
3. Life is adaptive and self-optimizing.
5
5
4. Life is compartmentalizedâit needs cells, enclosures, vesicles.
5. Life uses molecules that are suited to water.
The list is easy enough to compile; the difficult part is discerning what may be missing from it, since all we know about life comes from a single exampleâlife on Earth. Curiously, each one of the five items taken separately can describe a nonliving system; life seems to need all five together.
The first attribute is obvious but very useful to the big picture because it is an absolute for any origin of life in our Universe. There is no other form of matter or system capable of ordered networks; chemical bonds can do wonders under natural conditions. We cannot exclude the possibility that extant life-forms might develop a system (or develop
into
a system) that is not based on molecules, but that is not the question I am asking. I am interested in the process by which life emerges and the environments that allow it; that is, I am interested in the cosmic transition from chemistry to life. People have described nonmolecular (nonbiological) life-forms (in, e.g., the writings of Hans Moravec, Ray Kurzweil, and Steven Dick), but always as derivative or evolving from biological predecessors and based, at least partially, on molecules and molecular bonds.
6
We humans may be capable of creating ordered networks in silicon, but this is the result of a long history of the development of technology, not of a lifeless planet's original condition.
into
a system) that is not based on molecules, but that is not the question I am asking. I am interested in the process by which life emerges and the environments that allow it; that is, I am interested in the cosmic transition from chemistry to life. People have described nonmolecular (nonbiological) life-forms (in, e.g., the writings of Hans Moravec, Ray Kurzweil, and Steven Dick), but always as derivative or evolving from biological predecessors and based, at least partially, on molecules and molecular bonds.
6
We humans may be capable of creating ordered networks in silicon, but this is the result of a long history of the development of technology, not of a lifeless planet's original condition.
Planets help make this possible by providing a physical screen against space radiation, through their bulk and their atmosphere. Planets provide stability over an average timescale longer than the development of chemical networksâyears. For example, interstellar molecular clouds (like any cloud) cannot provide a macro- or microenvironment that
is stable or protected from destructive cosmic radiation on such timescales, while planets can.
is stable or protected from destructive cosmic radiation on such timescales, while planets can.
The second attribute is also an absolute: a system at equilibrium is a dead system in which nothing happens; you need energy to accomplish anything. To assemble an ordered networkâchemical or notâyou need energy. You need energy just to keep it ordered, to keep the inside separate from the outside.
The third attribute is the most fascinating thing about Earth life, and probably of any lifeâit can adapt itself to both fast and slow changes. Charles Darwin's genius was to see the essence of that property, which is based in part on the process by which new and different organisms develop as a result of changes in genetic material. We call it evolution, or Darwinian evolution in his honor. Most scientists consider it so important that they would define life through it. Gerald Joyce of the Scripps Institute summarized a NASA committee proposal in 1994 in a famous short definition of life as “a self-sustaining chemical system capable of Darwinian evolution,” sometimes referred to as the “NASA definition.”
7
Even though Darwinian evolution is an obvious way
8
to achieve diversity in response to changing environmental conditions and interaction with other living forms,
9
strictly speaking, it is not the only one.
7
Even though Darwinian evolution is an obvious way
8
to achieve diversity in response to changing environmental conditions and interaction with other living forms,
9
strictly speaking, it is not the only one.
The alternative is the existence of environmental conditions that allow continuous creation of life-forms with no inheritable molecules, but still with random variations.
10
The burden to sustain such a biochemistry then rests on the existence of environmental conditions that allow the continuous
synthesis of life-forms. Evolution offers a simpler and more straightforward mechanism, and is very likely a universal attribute to different origins of life and different biochemistries.
10
The burden to sustain such a biochemistry then rests on the existence of environmental conditions that allow the continuous
synthesis of life-forms. Evolution offers a simpler and more straightforward mechanism, and is very likely a universal attribute to different origins of life and different biochemistries.
In the cosmic transition from chemistry to life there is no substitute for molecules and what they can do; only one of the essential attributesâthe second, describing energy dissipationâcan be worked out without molecules. Any of the others will be impossible to do without some sort of complex moleculesâusually large ones, called polymers. Polymers are very long molecules made of smaller units called monomers (see
Figure 7.1
). The basic polymers of life, called biopolymers, are proteinsâmade by linking together amino acids and nucleic acidsâmade from nucleotides.
Figure 7.1
). The basic polymers of life, called biopolymers, are proteinsâmade by linking together amino acids and nucleic acidsâmade from nucleotides.
We might not know which chemical networks and attributes are essential to life, but as long as we are certain that some of them are, then molecules become building blocks and we can't do without them. Moreover, even though there are millions of molecules, the toolbox for building them is very limited, so we can identify some fairly narrow constraints on what must be around for life to happen. This is a good thing, as it gives us a fighting chance to understand them.
As we've seen, life must exist on a scale at which both gravity and electromagnetism are noticeable forces, and life needs protection from the environment of space. Consider temperature. In the Universe there is a wide rangeâfrom a few degrees above absolute zero to several hundred million degrees.
11
The coldest places are clouds of gas away from stars in the outskirts of small galaxies. Many planets are very cold too. In our Solar System the dwarf planets Sedna and Eris are
more than three times farther from the Sun than Neptune, and their surface temperature never rises above a bone-chilling 30 K (â400°F). The gas giant planets that were photographed recently around stars HR 8799 and Fomalhaut are at similarly great distances from their parent stars. It is expected that many more planets are equally cold.
11
The coldest places are clouds of gas away from stars in the outskirts of small galaxies. Many planets are very cold too. In our Solar System the dwarf planets Sedna and Eris are
more than three times farther from the Sun than Neptune, and their surface temperature never rises above a bone-chilling 30 K (â400°F). The gas giant planets that were photographed recently around stars HR 8799 and Fomalhaut are at similarly great distances from their parent stars. It is expected that many more planets are equally cold.
At the hot extreme of temperature are the envelopes of massive stars and gas near the central regions of galaxies. We know the destruction tolerances of molecules, and most of the high-temperature range is off-limits. While a few very hardy ones, such as carbon monoxide, survive at high stellar temperature (in low densities, they can survive temperatures as high as 6,000 K), any molecule with more than three atoms needs temperatures lower than those found in stars. Gas near the central regions of galaxies won't work either. The X-ray and UV light emitted by stars, as well as the energy released during the explosions of supernovae, keep lots of the gas in galaxies hot too.
The very low temperatures have their problems tooâpolymers survive but lose chemical functionality. Just as we know from personal experience (and make use of, through refrigeration), chemistry happens very slowly if at all at very low temperature. So, there is a narrow range of temperatures in which large molecules thrive and complex networks of chemical reactions can take place. That temperature range is from about 100 K to 600 K if we feel generous, but much narrower if we consider Earth biochemistry as we know it today.
That is a remarkable conclusion. In a Universe where temperatures of millions of degrees are common from a cosmic
perspective, life is a low-temperature phenomenon. At these low temperatures there are only a couple of large long-lasting types of objects in our Universeâclouds of gas between the stars, called molecular clouds, and planets (as well as their satellites and other small planetary bodies).
perspective, life is a low-temperature phenomenon. At these low temperatures there are only a couple of large long-lasting types of objects in our Universeâclouds of gas between the stars, called molecular clouds, and planets (as well as their satellites and other small planetary bodies).
Access to energy is another important environmental condition. In the above temperature range, there are two benign, steady, and long-lasting sources of energy in moderate amounts: starlight and internal heat of planets. Being a certain distance away from a star, such as Earth is from the Sun, is an excellent place. The energy flow is steady and moderate, meaning that it matches the energy needs of large molecules without destroying them readily. To access this stellar energy, you do not have to be on a planet. On the other hand, planetary hydrothermal vents, such as those in Yellowstone National Park or Iceland, or on the ocean floor, are a good example of how internal heat can provide a similar source of energy, both locally and globally on a planet, no matter how far from the star. Planets cool slowly, especially larger super-Earths, and this source can be steady for a very long time. In addition to heat, vents provide a rich source of chemicals that can be used as a source of energy. Hydrogen sulfide, for example, a chemical poisonous to you and me, is harnessed by bacteria at vents through a process known as chemosynthesis to power their cellular machinery.
Other books
Swindled (Close Contact Book 1) by Megan Mitcham
The Enchanted Life of Adam Hope by Rhonda Riley
Change-up by John Feinstein
The Evolution of Mara Dyer by Michelle Hodkin
Suspicion of Rage by Barbara Parker
Maddy's Floor by Dale Mayer
A Brighter Fear by Kerry Drewery
The Chocolate Meltdown by Lexi Connor
The Doctor's Lady by Jody Hedlund
