The Red Market (19 page)
Authors: Scott Carney

With all that in mind, I felt ready on the second day of the study at 6:45 in the morning when I got my first dose of the drug. They gave me a small bowl of cornflakes, whole milk, and fifteen minutes to finish up before asking me to line up with a subset of the total group. We understood that the entire study was being staggered between placebos, middling doses, and high doses of Levitra. I made eye contact with Frank and smiled at him. He eyed the nurses’ station with practiced ease, like a race car driver analyzing a track.
The stick went easily and the pretty young nurse who drew the morning shift sent me on my way to the head matron, who was wearing a dour look. The matron was seated at a desk. Standing on her right was someone holding a flashlight. In front of them was a blue paper towel with a single pill on it and a glass of water.
“Put the pill on your tongue and drink the whole glass of water. Be sure the pill goes down. Hiding it in your mouth will disqualify you from the study.” I realized that Frank probably had similar tricks up his sleeve for getting through trials unfazed. I downed the pill and the woman searched my mouth with the light and had me move my tongue just to be sure.
The current formulation of Levitra is administered in 2, 5, 10, and, for the most severe cases, 20 mg doses. I got 30 mg. The high dose was meant to test the upper limits of human endurance to be sure that the millions who take the drug down the line aren’t poisoning themselves. For lab rats, however, testing the limits of poisoning is the name of the game. Perhaps 30 mg is enough to make someone’s penis fall off. Nobody wants that.
I eventually meet up with Frank and I ask him if he took the pill. He tells me that pros can definitely hide drugs, but it’s not worth it for what we’re taking.
“The ‘me too’ drugs are the safest. Nothing to worry about.” I almost trust Frank that there is little danger. It was just a subtle twist on Viagra. And both drugs are really just a blood-flow redirector. What harm could it do?
To get approval a drug needs to pass through three phases of clinical research trials. The most dangerous is Phase I, where a small group of volunteers take high doses of an experimental drug to test its toxicity on healthy patients. This phase represents the highest allowable dose that a doctor should prescribe. Phase II happens on a larger group of sick patients and tests its effectiveness in treating a specific condition; and finally the massive Phase III trials are the safest and ultimately decide the drug’s clinical application. Professional test subjects rarely venture out for anything other than the most dangerous and high-paying trials.
THE TRIAL IN MADISON
was Phase I, and it didn’t take me long to realize that I was testing the upper limits of human endurance of erectile interactors. Within an hour my head started throbbing like it had been split down the middle. I fell into bed and kept the lights low. Finding the maximum permissible level means that the clinicians have to constantly skirt the line of safety, ratcheting down the dose only after inching it into the danger zone. In the hallways, beneath unforgiving fluorescent light, I could hear one of the other lab rats puking. He vomited into a toilet for a half hour, while nurses behind a Plexiglas barrier monitored his progress.
He asked for an Advil, but the nurse replied through an intercom that she needed to get permission from her boss before she could give him any treatment. She didn’t want to skew the data. Permission for the headache reliever made its way down the chain of command only after three hours.
Of the men in the study only two didn’t get headaches, putting the upper threshold of practically using this erectile dysfunction drug below 30 mg. The waiting room was full of both headaches and erections—not a particularly sexy combo.
I was still scheduled to come back for two more weekends, but as I made my way to the front exit, a nurse handed me a check at a reduced amount and said that they wouldn’t need me in the coming weeks. They didn’t tell me whether or not it was because the data my body provided didn’t meet their standards, or whether they’d just rather have fewer patients exhibiting splitting headaches on the official FDA filings. But I took the money. In an e-mail after the study, Frank wrote to me that sometimes it’s best not to acknowledge your symptoms if you want to collect the full amount. Frank had managed to complete the study for the full fee, and headed back down to Miami for a late summer month off.
I wondered whether I would really want to make a living testing solutions to erectile dysfunction. While the risks were likely minuscule, what was I going to get out of it other than a check and headache? And what is the purpose of having yet another Viagra knockoff on the market?
When I left my stint as a clinical laborer, I returned to the world of the uninsured and unemployed and started looking for another way to make a living. Like all lab rats, my duties were done the minute the meat of my body finished processing the drugs. I started to think about working in India. I had a master’s degree. Perhaps I could run an abroad program for college students.
It turned out that I wasn’t the only one looking for work abroad.
WHILE THE TESTING PROTOCOLS
mean that drugs on the market are safe and vetted as thoroughly as possible, getting approval is a long and expensive process that can easily cost as much as a billion dollars. Even then, final approval is not a sure thing.
While it’s true that blockbuster drugs like Viagra or an elite cancer therapy can easily offset the investment, drug developers feel pinched by the costs of drug testing in America and Europe. And yet for twenty years after the loss of prison test subjects the drug business bore the added expense. A new era dawned in the 1990s in the form of huge investments in biotech start-ups and public offerings on international stock exchanges that turned the pharmaceutical business into a high-profit/high-stakes roulette game.
Biotech companies and drug developers were increasingly led by MBA-holding boards of directors, not the scientists and clinicians who had a vested interest in patient outcomes. Speculating investors could back a company cheaply and wait for promising clinical trial results that would double a company’s stock price overnight and make millions for investors even if the drug was ultimately a dud and failed during later phases of the regulatory process.
The IPO mentality meant that a drug’s lifesaving properties were pegged to a bottom line. While blood-pressure regulators and treatments for hypertension and erectile dysfunction have seen a boom, others areas of research that are not as profitable have become less funded.
So many drug trials were running in the 1990s that pharmaceutical companies found themselves in over their heads and unable to cope with the load. They needed specialized help to manage the demand for drug data. Instead of doing all studies in-house and under the supervision of a university or research hospital, a slew of independent contract research organizations, or CROs, emerged that melded mercenary managerial skills with clinical sophistication. They were able to offer industrial levels of clinical testing and specialized in mass-market trials. All a scientist had to do was come up with an idea and testing protocol, and outfits like Premier Research Group in Philadelphia or Covance in Madison, Wisconsin, would run a prepackaged clinical trial off-site.
At first most contract research organizations were located in university towns where students in need of quick cash could sign up for studies. The only problem was that there weren’t enough students for the number of trials. CROs began to migrate to poor areas of cities, where they could easily attract low-income residents much like the blood business did in the 1950s. Since all they were responsible for was the data, CROs could cut costs in the same way as any other corporation: by finding ever cheaper sources of labor. Today CROs dot the US-Mexico border towns, attracting migrants into the testing facilities. Between 1990 and 2001 the office of the inspector general noted that there was a sixteenfold increase of clinical trials being conducted in low-income areas. They predicted that the number would double by 2007.
The estimate proved wrong. If anything, the number of CROs active in America has actually been decreasing. The inspector general failed to account for globalization. Data collection can easily be outsourced to foreign countries with looser ethical standards, lower operating costs, and lower per-capita incomes. A 2004 study conducted by Rabo India Finance calculated that outsourcing trials to India or China would cut the overall price of a drug trial by 40 percent. By 2005 the twelve largest pharmaceutical companies conducted half of their twelve hundred clinical trials in the United Kingdom, Russia, India, and China.
20
It’s a fortunate situation for American drug developers, not only because they save money, but because drug trials are able to overcome the major problem with professional lab rats back home. If they set up shop in areas where patients have had little prior access to health care, they can almost guarantee that their test subjects are treatment-naive. In large part because of their governments’ inability to extend health care to their citizens, both India and China have massive populations that can provide a natural human baseline of no prior treatment, even for critical conditions. By 2010 India was reaping the rewards of its overall treatment naïveté to the tune of $2 billion a year.
In India, “not only are research costs low, but there is a skilled workforce to conduct trials,” says Sean Philpott, former executive editor of the
American Journal of Bioethics
and current chair of the EPA’s Human Studies Review Board. The surge in willing subjects, however, raises similar questions that outlawed the prison studies in America. “Individuals who participate in Indian clinical trials usually won’t be educated. Offering one hundred dollars may be undue enticement: They may not even realize that they are being coerced,” he says.
The situation is similar to the pressure to sell kidneys in Tsunami Nagar. The same people who enroll in clinical trials in India belong to the same socioeconomic class as the people who are taken advantage of by kidney brokers, surrogate homes, and blood thieves. The surveillance and coercion between the markets are eerily familiar. Since the Indian Drug Control General, which plays an FDA-like role, generally has poor oversight, it is tempting for pharmaceutical companies to skirt ethical norms in order to create better data sets. There have already been mistakes.
In 2004 the Drug Control General of India investigated two high-profile biotech start-ups in Bangalore—Shantha Biotech and Biocon—for conducting an illegal clinical trial for genetically modified insulin. Eight patients died. Both companies failed to even ask for informed consent, let alone take measures to minimize the danger to patients.
In another incident, Sun Pharmaceuticals convinced four hundred doctors to prescribe Letrozole, a breast cancer drug, as a fertility treatment. They were hoping to get the drug approved for a secondary use (and double or triple sales), but failed to tell the patients they gave the drug to that they were enrolled in an experiment.
While the women reported no serious side effects, it had the potential for disaster.
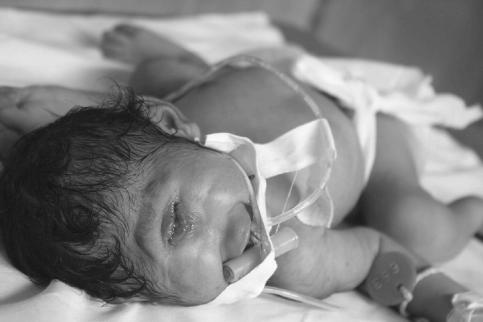
This child’s birth certificate reads “Baby of Gomathi” because her family refused to name her after she was born with a severe craniofacial defect known as cyclopia. The staff of Kasturba Gandhi hospital in Chennai wrote that the rare genetic disorder could have been the result of a botched fertility treatment with the drug cyclopamine. Cyclopamine was at that time being tested in the United States as a treatment for cancer. One year before this photo was taken pharmaceutical companies tested another anticancer drug as a fertility treatment in an unregulated trial of several hundred pregnant women. While cyclopamine is available for sale in India, no company admits to testing it in India.
And it may not have been the only time that a cancer treatment was tested on women who were pregnant or hoping to get pregnant. Two years after the Letrozole trial while I was living in Chennai, I reported for
Wired
News
that a child was born with severe facial distortions caused by a rare genetic disorder called cyclopia. The condition fuses the left and right hemispheres of the brain, and in this case resulted in a single eye in the center of the baby’s forehead, hence the name. When I visited Kasturba Gandhi Hospital where the child was born, the staff told me that the mother had told the hospital she had been trying to get pregnant for several years and was given an unknown drug by a local fertility clinic.
In a confidential report that I was allowed to read, the hospital administration wrote that the mother may have been given an experimental anticancer drug called cyclopamine. In my research I discovered that cyclopamine was currently undergoing clinical trials in the United States. The compound, derived from the North American corn lily, had long been used by Native Americans as a contraceptive and pain reliever. In the 1950s American herdsmen noticed that pregnant goats who had been eating corn lilies gave birth to an entire generation of one-eyed kids.
21
Further testing of the corn lily showed that the chemical cyclopamine blocks a genetic pathway crucial to the development of the brain and prostate cancer.
