What is Life?:How chemistry becomes biology (17 page)
Read What is Life?:How chemistry becomes biology Online
Authors: Addy Pross

Second, it seems logical to suggest that if life did start off simple, then life’s fundamental nature would become more understandable by examining earlier, and therefore
simpler
prototypes. An analogy may make this clear. If we want to understand what an airplane is, as well as the underlying principles that enable these modern behemoths to take to the air, then examining a fully equipped Boeing 747 will not be the most productive way to proceed. A Boeing 747 is an immensely complicated entity composed of some 6 million individual parts and over 200 kilometres of wiring, so figuring out the relevance of each and every part to the whole, and uncovering the basis for its flying capability, would be overwhelmingly difficult. Some of those parts, for example, passenger TV screens, steward call buttons, ovens for heating food, etc., wouldn’t be particularly
relevant to its flying capability. So where is one to begin? If you want to figure out what an airplane is, and the principles governing its flight, you’d be much better off examining an earlier simpler airplane, say the Wright brothers’ 1903 prototype or some other simple equivalent, where the number of components is a tiny fraction of that in the Boeing, and one in which every component plays an important, if not critical role in enabling that entity to become airborne. And that’s where systems chemistry comes in—by examining the workings of simple replicating systems and the networks they generate, we are attempting to do the equivalent of examining the Wright brothers’ airplane rather than a Boeing 747.
Of course the bottom-up approach toward resolving the life issue assumes that life
did
start off from simple beginnings and that a process of complexification from that simple beginning did take place. As discussed in
chapter 5
, that is the generally accepted view. It is the
nature
of that process that continues to be a source of intense debate, rather than whether the process took place. But, as we will shortly see, the emergent area of systems chemistry will also provide additional empirical support for that assumption. The goal of this chapter is therefore ambitious: to demonstrate that the study of systems chemistry can lead to the smooth merging of living and non-living systems, thereby offering a unifying framework for chemistry and biology. Such unification would be of considerable value as it would place biology within a broader chemical context. Indeed, if successful, that endeavour could provide fundamental insight into the ‘what is life’ question as it could offer a description of living systems in
chemical
rather than
biological
terms. So despite recent misgivings regarding the reductionist methodology as applied to biological systems, we will attempt to show that reduction in biology is alive and kicking (no pun intended!). In addition, a not insignificant side benefit would be to demonstrate that systems chemistry can throw light on the origin of life problem, at least in an
ahistorical
sense, by uncovering the
principles
that would have enabled inanimate matter to complexify in the biological direction toward life.
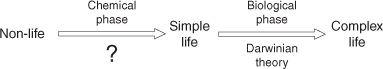
Fig. 6.
Two-phase (chemical and biological) transformation of non-life into complex life.
Let us then begin our discussion with the traditional view for the transformation of non-life into complex life. This can be represented as a two-stage process as illustrated in Fig. 6.
The first stage, the so-called chemical phase (termed abiogenesis, meaning the process by which life emerged from non-life) is where the never-ending debate and controversy lie. In the context of Fig. 6, a simple life form would mean that the system would possess what many would argue would be the most significant characteristic of living things—the ability to replicate and evolve in a self-sustained way. Indeed, having reached that critical point, the system would be considered biological in nature and its subsequent transformation into more complex life—single-celled eukaryotes and multicellular organisms—would have been governed by that momentous and earth-shattering theory that was proposed just 150 years ago, Darwinian evolution. So the conventional wisdom is that we are facing a two-stage process whose first stage is highly contentious and uncertain, while the second stage, in scientific circles at least, is in broad terms now unshakeable.
Let me now drop the bombshell, at least for many in the field. The so-called two-stage process is not two-stage at all. It is really just
one single continuous process.
If true that statement has quite profound consequences. First it must mean that hidden within Darwin’s theory of evolution—biological in formulation and application—a more fundamental, broader principle is at work, which must necessarily incorporate prebiotic systems, which by definition would be classified as non-living. In this chapter, I will attempt to justify the one-process assumption and explore some of its implications.
Why has the process indicated in Fig. 6 been considered a two-phase process until now? To put it bluntly—because of our ignorance. Knowing the mechanism of one phase and not knowing the mechanism of the other is a clear point of division and leads quite naturally to the separate classification. However, ignorance is not a useful basis for classification, so let me now try to justify the assertion that abiogenesis and biological evolution are in fact one single continuous process. And I don’t mean that in a trivial sense. It is obvious that if some prebiotic entity complexified into a simple living thing by some unknown mechanism, and then proceeded to evolve and diversify into the extraordinary range of living species, then whatever that early prebiotic process was, it could be thought of as continuous with the biological phase, at least in a temporal sense. But I intend the statement in a
non-trivial
sense—that the chemical process that led to the simple living creature and the biological process that subsequently carried on from there are one single process in a
chemical
sense. That is in fact exactly what recent studies in systems chemistry have been telling us. Let us review the empirical evidence.
In
chapter 4
, I described the molecular replication reaction of an RNA molecule carried out by Sol Spiegelman in the 1960s. We saw
that molecular replication is a chemical reality and can take place in a test tube, and not just in the highly regulated and specific environment of a cell. Recall, however, that Spiegelman also discovered that the population of replicating RNA molecules can evolve.
27
Over time the initially long chain RNA molecule evolved into shorter RNA chains. Shorter RNA molecules which replicated faster, out-replicated the longer ones, driving those longer ones to extinction. So what is termed natural selection within the biological world is also found to operate in the chemical world. The conclusion is highly significant. The causal sequence: replication—mutation—selection—evolution, normally associated with the biological world, in fact the s
ine qua non
of biology, is also clearly evident at the chemical level. That landmark work was carried out over forty years ago and since then the phenomenon of molecular evolution—evolutionary-like behaviour at the molecular level—has been observed by a growing number of researchers. Accordingly, the generality of evolutionary processes within replicating entities at the molecular level is now well documented and experimentally uncontroversial.
But the chemistry-biology nexus runs much deeper. Ecology is an established branch of biology and would seem to be quite unrelated to chemistry. However, as Gerald Joyce, the remarkable Scripps biochemist, reported in 2009, there is an intimate connection between the two.
53
A key ecological principle, termed the
competitive exclusion principle,
states: ‘Complete competitors cannot exist’ or, expressed in its positive form: ‘Ecological differentiation is the necessary condition for coexistence’.
54
What that principle teaches us is that two non-interbreeding species that occupy the same ecological niche (which just means that the two species compete
for the same resources) cannot coexist—the one that is better adapted to that niche (i.e., is fitter), will drive the other to extinction. Of course, if the two species feed off
different
resources then coexistence
is
possible. This basic ecological principle is classically illustrated by Darwin’s finches—one of the best-known examples of evolutionary theory. On the Galapagos islands, where Darwin visited in 1835, one can find a variety of finches that differ in the size and shape of their beaks. These different finch varieties, all of which derive from a common ancestor, evolved over time so as to exploit available resources more effectively. In doing so one type of finch—ground finches—evolved strong beaks which are effective for cracking nuts and seeds, while another type—tree finches—evolved sharp pointed beaks which are adapted for eating insects. The point is that these distinct varieties of finch can coexist because each is adapted to feed off a different resource, and in that sense provide a good example of the competitive exclusion principle.
But here is where the chemistry-biology connection comes in. Gerald Joyce discovered that this quintessentially biological principle also operates in chemistry.
53
Joyce found that when two different RNA molecules, let’s call them RNA-1 and RNA-2, were allowed to replicate and evolve in the presence of some essential substrate they were unable to coexist. RNA-1 turned out to be the more effective replicator with that substrate, and as a consequence it drove RNA-2 to extinction. If a different substrate was employed, one that RNA-2 was able to exploit more effectively, then the result was reversed. Now it was RNA-1 that was driven to extinction, as RNA-2 was the more effective replicator in the presence of that other substrate. Those chemical results are precisely in line with the predictions of the biological competitive exclusion principle. Since
both replicators relied on the presence of a particular substrate in order to replicate, the two molecules were unable to coexist—the faster (fitter) replicator drove the slower one to extinction.
But a more interesting and quite remarkable result was to follow: when the two RNA molecules were allowed to replicate and evolve in the presence of not
one,
but
five
different substrates, the two RNAs
were
able to coexist, but in an unexpected way. Initially the two RNA molecules utilized all five substrates in varying degrees in order to replicate. After all, all five were present and therefore all five could be utilized to some extent. But here is the punch line: over time each RNA molecule evolved so as to optimize its replicative ability with respect to
different
substrates. RNA-1 evolved so as to optimize its replicative ability with just
one
of the five substrates, while RNA-2 evolved so as to optimize its replicative ability with another of those five substrates. As a result, the two RNAs
were
now able to coexist.
In this beautifully designed experiment, which explored the characteristics of competing molecular replicators, the two RNA molecules were found to mimic the behaviour of Darwin’s finches precisely! Each molecule evolved to exploit a particular substrate efficiently, just as Darwin’s finches had evolved beak size and shape to suit the nature of the resource. That spectacular result, in which molecular replicators mimic biological ones (actually vice versa—molecular replicators preceded the biological ones), speaks loud and clear for a strong chemical-biological connection. Darwin’s finches are merely doing what certain molecules started doing billions of years ago.
Finally, let me show that complexification of the special kind normally found only in biological systems can also be discerned at the chemical level, and so provides yet another link between
chemical and biological replication processes. We have already discussed the fact that complexity is the very essence of biology. In fact, over an evolutionary time frame it is quite evident that complexity has continually increased from relatively simple systems to more complex ones. The earliest life forms that emerged, perhaps 4 billion years ago, were simple cells, prokaryotes (meaning that the cells lack a nucleus and other organelles). But after a further 2 billion years of evolution, eukaryotic cells emerged, in which membrane-bound organelles, including the cell nucleus, can be found. And some 600 million years ago another evolutionary transition involving further complexification took place, the one in which multicell organisms—plants and animals—appeared.
55
The evidence on this score is therefore unambiguous. Over the evolutionary time frame there has been a clear tendency for complexity to increase (though of course only among a small subsection of life, the multicellular eukaryotes; the vast majority of life, bacteria and archaeans, have remained happily simple). So within what we have labelled as the biological phase of Fig. 6, there is unambiguous evidence for a process of increasing complexity.
What can we say about the chemical phase of Fig. 6? In historical detail, almost nothing at all. But the essence of the transformation is quite clear. A molecular system, which we would characterize as non-living and relatively simple, somehow became transformed into a highly complex living cell, meaning the process involved was one of increasing complexity. As we have already pointed out, even the simplest living thing is highly complex. In other words,
both chemical and biological phases of Fig. 6 involved a process of continual complexification.
But how can this process of apparent complexification be understood at the chemical level?
