Why Is Milk White? (5 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Another trick is to curl the leaf up, like live oak leaves. This reduces water loss by reducing the wind and sun the leaf gets. Some leaves also have tiny hairs on them to reduce the water loss due to wind.
Yet another trick is to coat the leaves with a thick coat of protective wax, so the water stays trapped in the leaf. This protective coating of hard wax makes a glossy coat on the leaf, just like waxing a car.
An herbicide is a poison that kills plants. A weed-killer.
Many plants produce their own herbicides, so they can prevent other plants from using up their water, sunlight, or nutrients. The other plants can't grow underneath plants whose roots produce poisonous chemicals or whose leaves fall down and leak poisons into the soil.
The leaves of bay laurel trees produce natural herbicides that prevent other plants from growing up next to them. The flavor of bay leaves is in part due to these toxic (to plants) molecules. Walnut trees also produce natural herbicides.
Humans also make herbicides to control weeds. Some commercial herbicides kill almost any plant. Others are designed to only kill broad-leafed plants and not affect the grasses used for
lawns. Some of those work by acting like growth hormones when they are absorbed by the leaves. Plants with broad leaves absorb so much they grow faster than they can get nutrients, and so they die. Grasses have narrower leaves, don't absorb as much, and only grow a little more than they normally would.
Herbicides like Roundup use a chemical called
glyphosate.
Glyphosate prevents a plant enzyme from producing three amino acids that are critical to the plant. Since most plants need to produce these amino acids, glyphosate is a wide-spectrum herbicide that kills most plants it is used on.
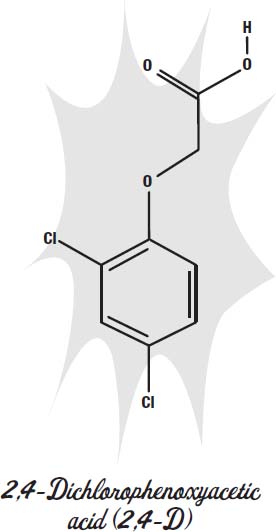
Selective herbicides are often synthetic molecules that mimic plant hormones. One selective herbicide is 2,4-D. It is inexpensive and kills broadleaf plants while leaving grasses mostly unaffected. 2,4-D mimics the plant hormone auxin. It is absorbed through the leaves of the plant and migrates to the fast growing parts of the plant, such as the tips of shoots. It stimulates the production in the plant of another hormone, ethylene, which can cause the leaves to fall off the plant if the dose is high enough.

Chemistry
What most people think of as chemicals are those things under the sink that are used to clean drains, polish silver, or disinfect kitchen counters. Those things with lots of long names in the ingredients list that are supposed to be kept out of reach.
But of course, everything that is made of atoms is a chemical. We use particular chemical reactions around the house to clean, bake, decorate, and glue. It is no surprise that most of Alexa's questions were about substances found in kitchen and bathroom cabinets.
Oil and water don't normally mix. When you get grease or oil on your hands, it is not easy to wash it off with water alone.
This is because water molecules bind to each other much more strongly than they do to the oil. The water molecules stick together and leave the oil molecules behind. This is the same reason water and air don't mix. Water and sand don't mix because the sand molecules stick to one another better than they stick to water.
The surface between water and oil, or between water and air, is made up of water molecules that pull on one another. We call this pulling
surface tension
. If we can make the water molecules pull on each other a little less, we can relieve some of that surface tension. This would allow the water to spread out more if you spilled some, instead of beading up into little droplets. We say that water with less surface tension is wetter than normal water, because it can more easily make things wet.
Soap is a special kind of molecule. It has one end that is strongly attracted to water. But the rest of the molecule is an oil. Oils are long chains of carbon atoms with hydrogen atoms attached to them. There might be anywhere from 10 to 30 carbon atoms in the long chain.
The end of the soap molecule that is attracted to water sticks into the water at the surface. The rest of the long chain sticks out of the water, because the water doesn't stick to the oily parts of the chains.
Oil molecules don't pull on one another as strongly as water molecules do. The surface of the water now has a film of oily ends of soap molecules instead of water molecules. The surface tension is now much lower than it was, and the water doesn't bead up. It can reach into all the crevices of your hands or in between the fibers of clothes in the wash.
The soap forms the surface between the water and the air. But it also forms the surface between the water and any oil or grease on your hands. The long oily chains in the soap stick into the oil, and the water-loving ends stick into the water. As you scrub your hands together, you flatten any droplets of oil or water trapped between them. This lets more soap molecules form a surface between the oil and the water. When the water or the oil tries to form round droplets again, the bigger surface wrinkles and forms many tiny droplets instead of big ones.
The droplets don't get back together to form big droplets again, because there is a soap film between them. So as you scrub, the
droplets get smaller and smaller. The tiny droplets, surrounded by soap molecules, no longer stick to your hands better than they do to the water. The water can now easily wash the tiny soap-coated oil droplets away.
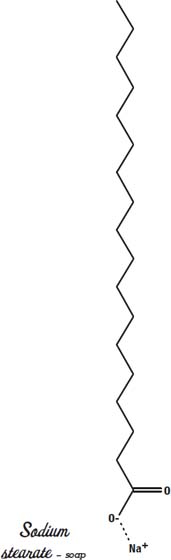
To make soap, take oil or fat and chemically add something that loves water to one end. Lye loves water. Lye is sodium hydroxide, a very powerful alkali. You can tell when a substance is strongly attracted to water because it releases heat when it reacts with water. When you add lye to water, the water gets very hot.
An
alkali
is the opposite of an acid. Acids and alkalis react strongly to produce salts. A fat or oil is three fatty acids attached to a molecule of glycerin. Adding lye to the fat allows the alkali to react with the fatty acids. The fatty acids are broken away from the glycerin and react with the lye to form soap. The glycerin is left behind.
The sodium part of the lye is now attached to the long chain of carbons that used to be a fatty acid. The sodium is still strongly attracted to water molecules, and the fatty acid end of the soap molecule ends up on the other side of the water surface, either in the air or in the oil.
If air is blown into soapy water, the soap molecules will quickly arrange themselves so that the fatty end is in the air bubble and the sodium end stays in the water. The bubble rises and meets the layer of soap at the top surface of the water. This surface has all of the fatty ends of the soap sticking up into the air.
As the bubble rises up, there are now two soap surfaces, one with the fatty ends of the soap facing the air outside and another with its fatty ends facing the air inside the bubble. The sodium ends of the soap hold on to the very thin layer of water between the two soap film surfaces. This is what soap bubbles are made of.
Shaving cream used to be made by mixing soap and water together using a shaving brush. The many bristles of the brush acted like a whisk to make lots of tiny bubbles.
Shaving cream in a can is a mixture of oils and soaps combined with a propellant made from propane or butane gas. The gas is under pressure (so much pressure that the butane is a liquid in the can). When the user pushes the button on the can, the pressure forces out the soap, and the gas expands into bubbles. The tiny bubbles are so small and numerous that they form a stiff lather.
The chemicals that clean your clothes work a lot like ordinary soap (see
page 25
): they're made up of molecules that stick to oil or grease on one end and to water on the other end, allowing them to pick up dirt and grime and then be washed away. But unlike soap, these chemicals are specially formulated to avoid causing what we call soap scum.
Soap scum forms when soaps reacts with
hard water,
which is water that contains too much magnesium and calcium. Soap is a fatty acid chain with the acid end neutralized by sodium. Sodium has 11 electrons, and the one outer electron is only loosely held by the atom. Its other 10 electrons are held very tightly by the sodium atom's nucleus. Sodium loses its outer electron to the oxygen atom in the fatty acid, which pulls strongly on electrons. The oxygen becomes negatively charged, the sodium is left positively charged, and opposites attract, so the sodium hangs around the fatty acid.

Right after sodium with its 11 electrons comes magnesium with 12 electrons. Magnesium has two outer electrons it can lose to fatty acids. So when magnesium reacts with soap, it replaces the sodium, hanging on to two fatty acids instead of one. Calcium also has two outer electrons, and it does the same thing. Unfortunately for people who use soap, the resulting molecule does not dissolve in water and instead forms soap scum. The scum sticks to clothes and hands and forms a dirty ring in the bathtub.
Chemists found out a long time ago that they could change the acid part of the fatty acid from a
carboxylic acid
to a
sulfuric acid
, and the result was a molecule that could dissolve in hard water, so it doesn't form soap scum.
Molecules like these are called
detergents.
You use them to wash your clothes, and you use them to wash your hair. Even some bars of “soap” are actually detergent bars.
You have seen how chemists can make better cleaning molecules than soap by changing part of the molecule so that it can dissolve in hard water. But there are other chemicals in cleaning products that can help in other ways.
Bleach is used to remove stains and kill germs. Bleach releases oxygen, which reacts with the stain or the germ. In effect, it slowly burns them. One kind of bleach is hydrogen peroxide, which can bleach clothes and disinfect cuts and scrapes but is also used to bleach hair.
Phosphoric acid is added to some cleaning products to dissolve hard-water films on glass and metal surfaces. It removes lime scaleâthe chalky substance left behind on coffeepots as hard water is boiledâby dissolving the calcium and magnesium compounds that form it. Phosphoric acid is a strong acid, but in dilute form it is added to cola drinks to give them a tart taste. Acids taste sour.
