Alien Universe (26 page)
Authors: Don Lincoln

While the date of the formation of life on Earth is an ongoing topic of debate, the period of about 3.5 billion years ago is a credible position, and the evidence grows increasingly stronger after about 2.7 billion years ago. Studies of the isotopic composition of early rock suggest that before about 2.4 billion years ago, there was very little oxygen in the atmosphere. However, at 2.4 billion years ago, the amount of oxygen in the atmosphere began to rise. The source of the oxygen was presumably early photosynthetic bacteria. For about half a billion years, the iron in the ocean absorbed oxygen and settled out on the ocean floor. This process went on until the iron was entirely used up and is the source of the iron mines we now exploit.
Once the iron was used up, the oxygen in the atmosphere began to rise
much more rapidly. As I mentioned, the source of oxygen was photosynthetic bacteria that had existed since the earliest forms of life, but, given oxygen’s reactive side, the oxygen was quickly bound to other substances in the ocean and eventually on the land. However, once these oxygen-loving materials in the sea and on the land were saturated, the oxygen concentration in the atmosphere increased. As the concentration of oxygen in the atmosphere grew, it encountered ultraviolet light from the sun. This led to the formation of ozone, which shields the Earth’s surface from ultraviolet light (and makes land-based life possible). Without ozone’s protection, the ultraviolet light would sterilize the surface of the planet, just like we use ultraviolet light to sterilize surgical instruments and to kill algae and parasites in fish tanks.
About 800 million years ago, the amount of oxygen in the atmosphere began to rise rather rapidly. This increase in oxygen is an oft-cited contributor to the origins of multicellular life (and, especially relevant to the idea of Aliens, animal life). The oxygen provided a large reservoir of a substance in the atmosphere that was an excellent acceptor of electrons and whose use in respiration and metabolism could generate lots of energy.
So oxygen is ubiquitous on Earth and plays a central role as part of all animals’ energy budget. The question when we think about Aliens is “is oxygen
necessary
?” We know of life on Earth that uses other substances as electron acceptors, with ferric iron, nitrates, sulfates, and carbon dioxide to name a few. However, these alternative forms of respiration are found in microbes, not multicellular animals, suggesting that the benefits of oxygen respiration are substantial and that evolution will likely nudge biochemistry in that direction if possible.
Even on Earth, the mechanism whereby oxygen is used to give energy to organisms isn’t a simple process but rather a multistep affair. Therefore it is possible that on a planet with an anoxic environment, evolution would invent a multistep process to get the required level of energy necessary to support Alien life. However, given the benefits of oxygen, it seems plausible that life would eventually find out a way to exploit it if it is present. This brings us to the next point.
Chemical Abundances
The chemistry we have been discussing is partially academic at this point. For instance, it may well be that carbon is the perfect atom from which to build life, but, if there is no carbon around, then it won’t be used. Similarly, if there is no oxygen present, it makes it kind of hard to use it to breathe. So we need
to add to our knowledge which elements are most present in the universe. To understand how certain elements are more or less common, we need to understand their origins.
Current theory is that the universe began just shy of 14 billion years ago in a cataclysmic event called the big bang. While the physics of the big bang is a fascinating topic, for our purposes, we merely need to know that the universe was once so hot that atoms couldn’t exist; indeed individual protons and neutrons couldn’t form, as the temperatures didn’t allow them to coalesce out of the bath of energy and subatomic particles that existed at the time.
As the universe expanded, it cooled in a way that is analogous to more familiar explosions, and very early in the history of the universe, protons and neutrons came into existence, followed by the elements hydrogen and helium. For all intents and purposes, no other elements existed. Following our discussion above, life couldn’t possibly form in that universe. Helium doesn’t form molecules, and hydrogen makes simple molecules consisting of two atoms. If that were the whole story, we wouldn’t be having this discussion. There must be more we need to consider.
Every morning as the sun rises, we are reminded of a seemingly trivial, but important, thing. The sun is bright and gives off heat. It does this because very dense collections of hydrogen and helium can undergo nuclear fusion. And nuclear fusion is one of the purest forms of scientific magic mankind has ever encountered and understood.
In medieval times, early scientists called alchemists were obsessed with the transformation of materials from one form to another; of “base metals” (e.g., lead) into gold. While there is no doubt that modern chemistry owes a debt to the early alchemists, they were doomed in their quest to transform one element into another. Such a goal is simply beyond the ability of chemical reactions.
However, the nuclear fusion of stars accomplishes just that. The nuclei of light elements are combined, forming heavier elements. In these stellar foundries, hydrogen and helium are forged into oxygen, carbon, nitrogen, silicon, and all elements lighter than iron. Standard, stellar-based, nuclear fusion fails to create heavier elements.
As it happens, some stars burn fast and furious and end their lives in a spectacular explosion called a supernova. In nearly a blink of an eye, these stars die, experiencing heat and nuclear reactions that dwarf those in more complacent stars. With their death, they form even heavier elements … even the creation of gold that eluded the ancient alchemists. This is the reason that
Carl Sagan so often stated that we are all “starstuff.” Without stars, life and even planets would not be possible. In fact, the first stars formed when the universe couldn’t have planets. The ingredients of planets just didn’t exist. But, in their death, the early stars spread a complex brew of elements across the cosmos. These elements mixed with the existing hydrogen clouds and formed subsequent stars.
Our sun is a second- or third-generation star, having formed about 5 billion years ago. At the time of the sun’s birth, the universe had had 9 billion years for earlier stars to manufacture the other elements of the periodic table. The elements present when our solar system came into existence formed the reservoir from which the planets and any possible life must be composed.
Figure 6.7
shows the relative abundances of the thirty lightest elements in our solar system. Hydrogen and helium make up 99.9% of the matter in the solar system, but from the remaining 0.1%, the planets coalesced. Of the remaining elements, carbon, oxygen, and nitrogen (the elements of organic chemistry and life as we know it) are the next most available. The relative abundance of all of the elements is in pretty good agreement with our understanding of how they are formed in the stellar furnaces in which they were created. Silicon, which is carbon’s chemical cousin, is present in quantities that are about 10% that of carbon. So, a naïve interpretation of this graph might make you say, “well, yeah, it makes sense that life would be made of carbon, since there’s more of it.” Conversely, it doesn’t take a lot of thinking to say, “Hey wait a minute. If carbon is so much more prevalent than silicon, why is Earth a big rock (i.e., silicon dioxide) rather than being made mostly of carbon? What gives?”
And, of course, that is an interesting question. The question of relative abundances of elements in the solar system tells us a lot, but life couldn’t form from the elements inside the sun. It likely had to form on (or under or in the atmosphere of) the surface of a planet. So the correct elemental abundances to consider would be those on the surface of the planet. (The same logic that shows that the chemical composition of the star is only marginally relevant also rules out the molecular makeup of a planet’s core as an important consideration. It is the makeup of the planetary crust that defines the reservoir of elements from which life can be formed.) I use the word “planetary” in a generic sort of sense. Life could have formed on moons of planets that are themselves sterile. We’ll see in a short while the reason silicon doesn’t play a central role in earthly life.
At this point, we begin to see how difficult it can be to generalize the discussion of chemistry and Alien life. After all, the environments on the various planets and moons in our own solar system are extremely diverse. The gas clouds of Jupiter are quite different from the scorched surface of Mercury, the frozen wastelands of Europa, and our own familiar Earth. It is this diverse range of environments that makes it so hard for astrobiologists to decide where to look for life.
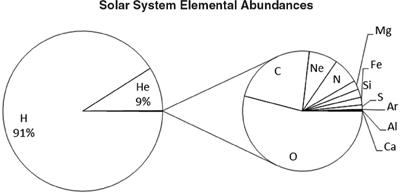
FIGURE 6.7
.
The distribution of elements in our solar system is totally dominated by hydrogen (H) and helium (He). Even the relatively common carbon (C) and oxygen (O) make up less than 0.1%.
However, we need to remember that we are interested in Aliens, rather than alien life per se. Aliens are creatures with sufficient intelligence to employ tools and someday compete with humans for galactic domination. Thus it is difficult to imagine a life-form suspended in the clouds of a gas giant as an Alien. It is much easier to imagine a creature on a rocky planetary object as a competitor. For one thing, access to metals is very important for manufacturing most tools and weapons. In a frigid environment, other materials might serve the same purpose. But in any case, the surface of a rocky planet is probably the relevant elemental reservoir to build our discussion of Alien life around.
We can start with the chemical makeup of the Earth’s crust as a baseline. This is given in
figure 6.8
. There are striking differences in the Earth’s elemental makeup compared with the solar elemental abundances, underscoring that the details of planet formation are critical. Hydrogen and helium are rare. We also see that the noble gases (helium, neon, argon, etc.) are noteworthy in their absence. These elements are gaseous and do not bind to other elements to form solids. Oxygen is the most present element, followed by silicon. This mix reflects the various rocks (feldspar, quartz, etc.) that make up the surface of the Earth. Carbon is very rare in comparison to silicon (a tiny fraction of a tiny fraction, compared with about a quarter of the Earth’s crust being made up by silicon). And this probably is telling us something significant. Even given the vastly larger amount of silicon available and the fact that both elements can create four bonds, life is formed from carbon. The ability to form four bonds is very important, but there are other considerations that must be taken into account when thinking about the chemical makeup of possible life. We will discuss at the end of the chapter silicon’s issues as a building block of life. (I know I’ve promised this more than once, but we need a bit more background to explore the limitations of silicon as the basis of life, as well as to introduce an innovative way to overcome carbon’s striking advantages.)

FIGURE 6.8
.
The elemental abundances of the Earth’s crust reflect the fact that it is made of rock, which has a very high silicon (Si) and oxygen (O) component. The pronounced differences between the elemental makeup of the Earth’s crust and the solar system as a whole highlight how accidents of planetary formation can significantly affect the chemical reservoir available to create life.
We will also talk a little later about the nature of liquid that forms life. On Earth, this liquid is universally water. As we wrap up our discussion of chemical availability, we can take a look at the elemental makeup of the Earth’s oceans. This is given in
figure 6.9
. Because our oceans are made of water (H
2
O), oxygen and hydrogen are the most prevalent atoms. Further, since most of the water on Earth is salty, it is unsurprising that sodium and chlorine, the elements that make up ordinary salt (NaCl) are present. The other elements are present if they can be tied up in molecules that are soluble in water.
