Alien Universe (25 page)
Authors: Don Lincoln

FIGURE 6.3
.
This is a couple of ways to represent how hydrogen atoms (H) combine to make a hydrogen molecule (H
2
). The electrons of the two atoms are shared between them. On the bottom, we see a shorthand, with the atomic symbol standing in for the atom and a long dash (–) to represent the bond.
However, the column that allows for the most intricate molecular structures is the carbon one. Carbon and other elements in that column can form four bonds. Continuing our exploration of bonding with hydrogen, a carbon atom bonded with four hydrogen atoms makes a methane molecule. In our analogy of arms, nitrogen has three arms, while carbon has four.
Carbon (like any atom) can connect with more than simply hydrogen atoms. It can combine with other carbon atoms, as well as all of the other atoms of the periodic table. Mind you, this is also true of the nitrogen and oxygen columns, but it is the ability to make four bonds that allows the most complex molecules to be created.
Figure 6.4
gives just a sense of the kinds of structures that become available when one has atoms that have this many bonding possibilities. These are the molecules of life on Earth.
Now you’ve probably already gotten ahead of me and thought, “But what about the other elements in that column?” After all, silicon can also form four atomic bonds. Is silicon-based life possible?
Certainly silicon atoms can compose complex molecules; however the situation is more difficult than simply replacing carbon atoms with silicon ones. As a simple example, consider the common carbon dioxide that we exhale as we breathe. Carbon dioxide is a gas, which makes it easy for the fluid (i.e., blood) in our bodies to transport it. In contrast, silicon dioxide is a solid, known by the more common name of “sand.” We will return to silicon-based life at the end of the chapter.

FIGURE 6.4
.
The different elements can participate in a different number of bonds, ranging from zero to four. The more bonds in which a specific element can participate has a large effect on the complexity of the molecules that can be formed.
Bond Strengths
While the number of bonds in which an atom can participate is a very important consideration, of equal importance is the strength of the bonds. The molecular and atomic world is a frenetic place, with constant motion being the norm. Due to simple heat, atoms vibrate, bounce into one another, and undergo a continuous stream of collisions. If the bonds aren’t strong enough, these atomic and molecular collisions could rip apart the molecules of life, just like a hard tackle in football can cause a fumble. Without a stable molecular environment, surely no life could exist.
We can understand this point in a visual way by considering one of those reality television shows where they come up with ridiculous competitions. Suppose this show is called “Togetherness” and the point is that two people are tied together somehow and they are to stay together for the entire season. If their connection fails, they are disqualified. Suppose one couple is tied together with ordinary sewing thread, while another is connected with the
kind of rope that mountain climbers use. It doesn’t take much imagination to realize that the couple connected by a thread has a serious disadvantage. Just in the day-to-day to and fro of life, with walking around, brushing one’s teeth, sleeping, and so on, something is going to break that thread. In contrast, there is very little that the rope couple will encounter that will cause them to be separated.
There are a couple of ways that atoms can be bonded together, but the strongest is called a “covalent bond.” In a covalent bond, some of the electrons in each individual atom are shared between the two atoms. In a sense, the two atoms sort of fuse together into a single molecular unit. And these bonds are really strong. To give a sense of scale, two hydrogen atoms can bond this way to form a hydrogen molecule. The bond is so strong that if you took hydrogen gas at room temperature and pressure, you’d need a volume of gas the size of the Milky Way galaxy to have a 50% chance of breaking apart a single molecule into its two constituent atoms. These molecules are
really
hard to break apart. If they weren’t, a volume containing that many atoms would have many broken molecules.
Getting back to the question of which atoms are most likely to have a significant role in life, we can ask if different elements can form stronger or weaker bonds. It turns out that the lower-mass elements can form much stronger bonds than the heavier ones. The reason is a little subtle, but luckily not too hard to understand. It all boils down to the degree to which the atoms overlap one another. The larger fraction of overlap, the more those two electrons are shared and the stronger the bond. This point is illustrated in
figure 6.5
.
This figure is simplified, but has some valuable features. Atoms consist of a nucleus and then a swarm of electrons around the outside. The electrons closest to the nucleus (or in the lowest energy states, if you’ve taken a chemistry class) are not generally available to form bonds, while the outer few electrons are. In
figure 6.5
, I’ve chosen to represent the core, noninteracting, portion of the atom as a black dot. The outer white circle is intended to represent the electrons available to form bonds. You’ll note that I drew a small and large atom. For both atoms, the thickness of the white area is the same.
I then graphically made molecules, by connecting two atoms together. To a degree, one can say that the atoms share the electrons in the region between the two atoms where the white areas overlap. This overlap region is indicated in gray. Now compare the gray region to the white region in small-atom molecules and large-atom ones. You see that in the small-atom molecules that the gray area is a larger fraction of the white area. Smaller atoms share their electrons with their neighbors a greater fraction of the time, which is the basis for the much stronger bonds in the lighter elements.
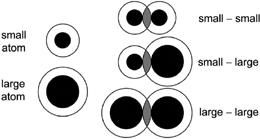
FIGURE 6.5
.
The strength of a covalent bond depends a lot on just how much the electrons from the atoms overlap. The larger the fraction of time they overlap, the stronger the bond. Here, the white area represents the electrons available for bonding, while the gray area represents the region of overlap. In smaller molecules, the gray area is a larger fraction of the white area.
These simple considerations show why it is somehow natural for life to be formed of carbon. Carbon can form four strong bonds with neighboring atoms, allowing the formation of complex molecules. Other light atoms cannot form as many bonds, reducing the complexity of the possible chemistry, while other heavy atoms cannot form as strong a bond, thereby reducing the probability that the molecules will be stable. Carbon is an optimum element for complex molecular chemistry.
It is, perhaps, unsurprising that we carbon-based life-forms would conclude that carbon was an ideal basis for forming life. This is called “carbon chauvinism.” We will return to this point when we’ve finished our overview of the important components of life and consider alternative chemistry.
Oxygen
All multicellular life on Earth uses oxygen as part of its respiration system, although this is not true of all forms of life. The role of oxygen is that it is a receptor of electrons. The movement of electrons is the source of the energy of life, so an element that can accept electrons is facilitating the flow of energy. Oxygen is a superlative acceptor of electrons.
Is the use of oxygen a necessary feature of life in the universe? Well, the answer is pretty clearly no, given that we know of life on Earth that uses other substances to breathe. In fact, we are quite confident that the first forms of life on Earth would have been killed by the presence of oxygen. So what is it about oxygen and why has it become such a ubiquitous presence on Earth now? Does the universal usage of oxygen by multicellular Earth life mean that oxygen breathing is universal?
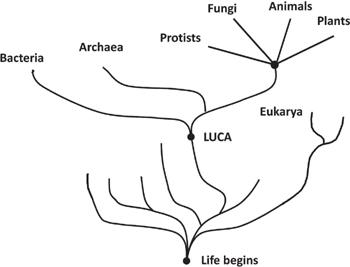
FIGURE 6.6
.
How it is believed that the first living organism began and underwent speciation is shown here. Eventually all of the early branches of life died out except for one organism that was the last universal common ancestor, or LUCA. This diagram shows only the most basic points, as cross-species genetic mixing is thought to have occurred when the organisms were simpler.
It doesn’t, of course, but it’s worth spending a little time learning about the essentials of the role of oxygen in the history of life on Earth. We don’t know very much about the first life on Earth. Life formed and many species evolved and became more complex. As is usual with evolution, some species thrived, while others became extinct. It is thought that one of these complex organisms is the parent of all existing species, while the others died out. This parent being is called the last universal common ancestor, or LUCA. A family tree showing how life might have branched out is depicted in
figure 6.6
.
Working backward from today, biologists are quite confident that mankind shared a common ancestor with chimpanzees. That common ancestor shared an even earlier ancestor with other primates. The primates shared a common ancestor with other mammals. Moving backward in time, we now believe that each of the domains, kingdoms, phyla, classes, and so on, mentioned
in the previous chapter originated from a common ancestor, whose descendants varied slightly and consequently set into motion the physical and biological differences we observe now in these different divisions of life. Each of the domains of Prokarya, Eukarya, and Archaea had a different common ancestor, although modern research suggests that Eukarya was formed by a mixture of earlier Archaea and Prokarya ancestors.
Taking the pattern one step further, there presumably was an organism who was the ancestor of all forms of life on Earth. Now this ancestor (the last universal common ancestor, or LUCA, mentioned above) was not the first form of life the Earth saw. Using comparative genetics and biochemistry, scientists have learned a lot about LUCA. For instance LUCA used DNA and a couple of hundred proteins to live. LUCA was already a very complex organism, quite different from the earliest form of life. It’s hard to know which adaptation from LUCA gave it the edge to survive and thrive, while all of its cousin contemporaries were doomed to extinction. But survive it did and here we are.
LUCA probably didn’t depend on oxygen for its respiration. While our understanding of LUCA’s biochemistry is incomplete, it seems to be true that iron was an important part of its metabolic pathways. This fact is pretty conclusive evidence that LUCA lived before the Earth’s atmosphere had a lot of oxygen in it. We know this as iron really loves to combine with oxygen into a form that is
extremely
insoluble in water. If there was a bunch of oxygen around, the iron would get gobbled up and pulled out of the ecosystem in the form of rust. As you’ve no doubt experienced, rust doesn’t dissolve and, once the iron is in the form of rust, it is unavailable for future use. In order for an organism to depend a lot on iron means that it must exist in an anoxic (low/no-oxygen) environment.
