Connectome (17 page)
Authors: Sebastian Seung

The studies of twins show that genes matter, but they do not explain why. Before I tackle the answer (or many answers) to this question, let me explain some things about genes.
Â
You can think of a cell as an intricate machine built from molecular parts of many types. One of the main types is a class of molecules known as proteins. Some protein molecules can be structural elements, supporting the cell like the studs and joists of a wooden house frame. Other protein molecules perform functions on other molecules, much as workers in a factory handle parts. Many proteins combine both structural and functional roles. And the cell is more dynamic than most man-made machines, as many of its proteins move around from place to place.
It's commonly said that DNA is the blueprint of life, because it contains the instructions that cells follow to synthesize proteins.
Just as DNA is a chain of nucleotides, a protein molecule is a chain of smaller molecules called amino acids, which come in twenty types. Each kind of protein is specified by a sequence of letters, but the alphabet contains twenty letters rather than the four used in DNA. This amino acid sequence is specified by a (mostly) contiguous string of lettersâa geneâin your genome. To produce a protein molecule, the cell reads the nucleotide sequence of a gene and “translates” this into an amino acid sequence to synthesize a protein. (The dictionary for translation is known as the genetic code.) When a cell reads a gene and constructs a protein, it is said to “express” the gene.
You started your life as a single cell, an egg fertilized by a sperm. This cell divided in two, and its progeny divided, and so on for many generations to produce the huge number of cells in your body. Every dividing cell replicated its DNA and passed on identical copies to its progeny. That's why every cell in your body contains the same genome.
Why then do a liver cell and a heart cell look different and perform different functions? The answer is that cells of different types express different genes. Your genome contains tens of thousands of genes, each corresponding to a different kind of protein. Each type of cell expresses some of these genes but not others. Neurons are arguably the most complex type of cell in the body, so it's no surprise that many genes encode proteins that are exclusively or partially devoted to supporting functions in neurons. This is a preliminary answer to the question of why genes matter for the brain.
Your genome and mine are almost identical, conforming almost exactly to the sequence that was found by the Human Genome Project. But there are also slight differences, and the field of genomics is developing faster and cheaper technologies for detecting them. Sometimes the differences reside in single letters, while other times a longer stretch of letters is deleted or duplicated. If a genomic difference alters a gene, we can make a guess about the consequences if we know the function of the protein encoded by the gene.
By now you're familiar with the idea that mental function is based on spiking and secretion. Both processes involve many kinds of proteins. You've already encountered an important kind, the receptor molecules that sense neurotransmitter. These sit in the outer membrane of a neuron, partially protruding from the exterior of the cell. (Remember the kid floating in the inner tube?) Earlier I described the binding of a neurotransmitter molecule with a receptor as being like the insertion of a key into a lock. The metaphor goes even further for some receptors, which are a combination of a lock and a door. A small tunnel threads through the receptor molecule, connecting the inside of the neuron to the outside, but it's blocked by a doorlike structure most of the time. When the neurotransmitter binds to the receptor, the door opens for an instant, and electrical current can momentarily flow through the tunnel. In other words, the neurotransmitter acts like a key that opens a door, allowing electrical current to flow between the inside and outside of the neuron.
In general, we use the term
ion channel
for any type of protein containing a tunnel that passes electrical current through the membrane. (Ions are the electrically charged particles that conduct electricity in aqueous solutions.) Many types of ion channels are not receptors. Some of them enable the neuron to generate spikes; others have subtler effects on the electrical signals traversing neurons. If your genome contains an abnormal DNA sequence for a receptor or ion channel, it could be bad news for brain function. A disease caused by a defective DNA sequence for an ion channel is called a “channelopathy.”
Malfunctioning ion channels can lead to the uncontrolled spiking that we call epileptic seizures.
There are other types of proteins that package neurotransmitter into vesicles, as well as proteins that help release the contents of the vesicles into the synaptic cleft when triggered by a spike. Other proteins help degrade or recycle the neurotransmitter in the cleft, preventing it from lingering too long or drifting off to other synapses. This list is only the tip of the iceberg; it does not do justice to the vast array of proteins that serve spiking and secretion. Defects in any of these proteins could lead to brain disorders.
The possibilities for malfunction go way beyond that, however. On top of their present-day effects, defective genes might have made their mark in the past, when they caused the development of the young brain to go awry.
Â
Roughly speaking, the brain grows and develops in four steps. Neurons are created, or “born,” through the division of progenitor cells, migrate to their proper places in the brain, extend branches, and make connections. Disruption of any of these steps can lead to an abnormal brain.
What happens if the creation of neurons does not proceed successfully? In the city of Gujrat in Pakistan, there is a shrine to a seventeenth-century holy man named Shua Dulah. For centuries, babies born with abnormally small heads have been left at this shrine. In Pakistan they are known as
chuas,
which translates as “rat people,” probably because their faces protrude in a somewhat ratlike way. The chuas are sometimes exploited by chua masters, who send them out to beg and then take the proceeds. The people tell various myths to explain the existence of chuas. One is the gruesome story that chuas are created by evil people who place clay or metal caps
around the heads of babies, thereby retarding the growth of their brains.
In reality, the chuas are born with the disorder of congenital microcephaly. In the purest form, microcephaly vera, the only abnormality appears to be reduced brain size at birth.
The cortex is smaller, but the pattern of folds
and other architectural features are roughly normal. Not surprisingly, given the smaller cortex, microcephaly vera is accompanied by mental retardation.
Researchers have found that defects in a number of genes (with names like microcephalin or ASPM) can cause microcephaly vera. These genes encode proteins that control the birth of cortical neurons. Defects in them reduce the number of neurons and cause microcephaly. Because there are two copies of every gene, it's possible to carry one defective copy without showing any symptoms; the single correct copy is enough to make the brain grow normally. But when two carrier parents each pass on a defective copy to their child, he or she is born with microcephaly. This event would normally be rare, but in Pakistan it happens more frequently because of the high rate of intermarriage between cousins.
(Since cousins are genetically related, it's more likely for them both to be carriers than it is for two people chosen at random.)
The second step of brain development, the migration of neurons to their proper places, can also be disrupted. In the disorder of lissencephaly (from the Greek roots for “smooth brain”), the cortex lacks the folds that normally give it a wrinkled appearance, and possesses other structural abnormalities visible in a microscope. The condition is usually accompanied by severe mental retardation
and epilepsy. Lissencephalies are caused by mutations in genes that control neuronal migration
during gestation.
These two steps in brain development occur in the prenatal brain. By the time a baby is born, the creation and migration of neurons are virtually complete. You may have heard that you were born with all the neurons that you will ever have. (There are only a few areas of the brain in which neurons still continue to be created after birth.) But this does not mean that brain development is over. Neurons continue to grow branches well after birth. This process is called the “wiring” of the brain, since axons and dendrites resemble wires. Axons have to grow the most, since they are much longer than dendrites. Imagine the tiny growing tip of an axon, known as a “growth cone” for its roughly conical shape. If a growth cone were blown up to human size, its travels would take it to the other side of a city. How is the growth cone able to navigate such long distances? Many neuroscientists study this phenomenon, and they've found that the growth cone acts like a dog
sniffing its way home. The surfaces of neurons are coated with special guidance molecules that act like scents on the ground, and the interstitial spaces between neurons contain drifting guidance molecules that act like scents in the air. Growth cones are equipped with molecular sensors and can “smell” the guidance molecules to find their destination. The production of these molecules and their sensors is under genetic control. That's how genes guide the wiring of the brain.
If axons don't grow properly, “miswiring” results. Consider the corpus callosum, a thick bundle of 200 million axons
connecting the left and right hemispheres of the cerebrum. In rare individuals, the callosum is either completely or partially missing. Fortunately, the impairments are much milder than in microcephaly.
Such miswiring could be caused by defects in many genes, including those that control axon guidance.
For most of its journey through the brain, an axon grows straight, like the trunk of a tree. Once the growth cone reaches its final destination, the axon starts to branch. Scientists have reason to think that this final branching might not be so tightly controlled by genes. If this is the case, the detailed branching pattern of a neuron is largely random, although its overall shape might be genetically determined. Likewise, trees in a pine forest look similar because they come from the same genetic plan. No two trees match exactly branch for branch, however, because growth also involves randomness and is influenced by environmental conditions.
As the wires of the brain are laid down, neurons connect with each other by creating synapses. I hypothesized earlier that the process of synapse creation is random, happening with some probability whenever neurons contact each other. There is also room for genetic control, because neurons of different types might recognize each other through molecular cues and “decide” on that basis whether to connect. (I'll talk later about neuron types.)
So the initial connectome produced by very early development appears to be largely a product of genes and randomness. Scientists are still studying their relative contributions. According to one theory, genes exert their influence mostly by controlling how the brain wires up. Genes roughly determine the shape of a neuron, the region over which it extends branches. If there is an overlap between the regions spanned by two neurons, there is potential for connection between them. But whether they actually connect is not determined by genes. At first, it depends on random encounters of branches within the genetically defined regions, and on random creation of synapses at these encounters. But as development proceeds, experiences also start to shape the connectome. How exactly does this happen?
Â
New synapses are created at a staggering rate in the infant brain. In Brodmann area 17 alone, over half a million per second
are produced between two and four months of age. To accommodate the synapses, neurites increase in both number and length. Figure 25 illustrates the dramatic growth of dendritic branches from birth to two years of age.
Â
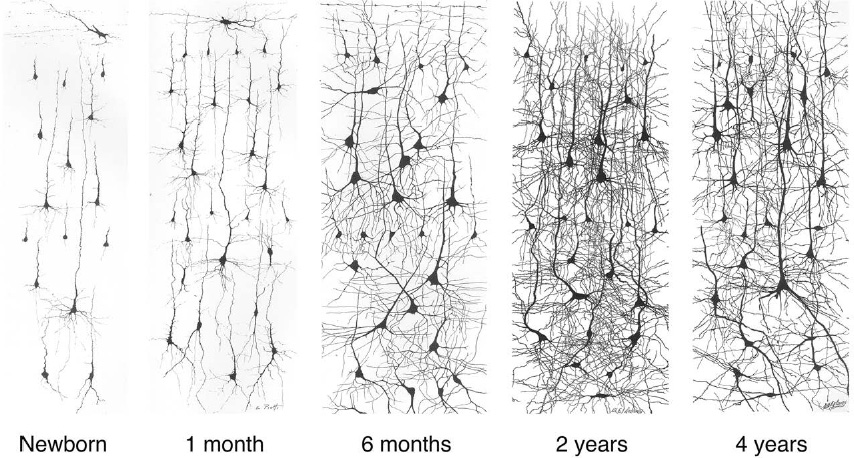 Â
Â
Â
Figure 25. Dendrite growth from birth to age two, followed by pruning
Â
I cautioned in Chapter 5 against thinking of adult learning as purely synapse creation. The same is true of the young brain, for development also
destroys
connections. When you were two years of age, you had far more synapses than you have now. By adulthood, the number of synapses has dropped
to 60 percent of its peak during the toddler years. A similar rise and fall holds for the branches of neurons. Dendrites and axons grow exuberantly at first, but some branches are later pruned away (compare the last two panels of Figure 25).
Why does the brain create so many synapses, only to destroy many of them later? Actually, many so-called creative acts are misnamed, because they involve both creation and destruction. When I'm writing an article, I focus first on getting all my thoughts out onto the page, even if the writing is embarrassingly bad. During this phase, the words increase in number. After a rough draft is complete, further rewriting or editing often shortens the piece. The final article ends up having fewer words than the draft. As the saying goes, perfection is achieved not when there is nothing left to add, but when there is nothing left to take away.
