Eight Little Piggies (41 page)
Read Eight Little Piggies Online
Authors: Stephen Jay Gould

The most impressive evidence for neutralism as a maximal rate has been provided by forms of DNA that make nothing of potential selective value (or detriment) to an organism. In all these cases, measured tempos of molecular change are maximal, thus affirming the major prediction of neutralism.
1.
Synonymous substitutions
. The genetic code is redundant in the third position. A sequence of three nucleotides in DNA codes for an amino acid. Change in either of the first two nucleotides alters the amino acid produced, but most changes in the third nucleotide—so-called synonymous substitutions—do not alter the resulting amino acid. Since natural selection works on features of organisms, in this case proteins built by DNA and not directly on the DNA itself, synonymous substitutions should be invisible to selection, and therefore neutral. Rates of change at the third position are usually five or more times as rapid as changes at the functional first and second positions—a striking confirmation of neutralism.
2.
Introns
. Genes come in pieces, with functional regions (called exons) interrupted by DNA sequences (called introns) that are snipped out and not translated into proteins. Introns change at a much higher evolutionary rate than exons.
3.
Pseudogenes
. Certain kinds of mutations can extinguish the function of a gene—for example, by preventing its eventual translation into protein. These so-called pseudogenes begin with nearly the same DNA sequence as the functional version of the gene in closely related species. Yet, being entirely free from function, these pseudogenes should exert no resistance against the maximal accumulation of changes by random drift. Pseudogenes become a kind of ultimate test for the proposition that absence of selection promotes maximal change at the neutral rate—and the test has, so far, been passed with distinction. In pseudogenes, rates of change are equal, and maximal, at all three positions of the triplet code, not only at the third site, as in functional genes.
I was inspired to write about neutral theory by a fascinating example of the value of this framework in assessing the causes of evolutionary rates. This example neither supports nor denies neutralism but forms a case in the middle, enlightened by the more important principle that random models provide simple and explicit criteria for judgment.
While supposedly more intelligent mammals are screwing up royally above ground, Near Eastern mole rats of the species
Spalax ehrenbergi
are prospering underneath. Subterranean mammals usually evolve reduced or weakened eyes, but
Spalax
has reached an extreme state of true blindness. Rudimentary eyes are still generated in embryology, but they are covered by thick skins and hair. When exposed to powerful flashes of light,
Spalax
shows no neurological response at all, as measured by electrodes implanted in the brain. The animal is completely blind.
What then shall we make of the invisible and rudimentary eye? Is this buried eye now completely without function, a true vestige on a path of further reduction to final disappearance? Or does the eye perform some other service not related to vision? Or perhaps the eye has no direct use, but must still be generated as a prerequisite in an embryological pathway leading to other functional features. How can we decide among these and other alternatives? The random models of neutral theory provide our most powerful method. If the rudimentary eye is a true vestige, then its proteins should be changing at the maximal neutral rate. If selection has not been relaxed, and the eye still functions in full force (though not for vision), then rates of change should be comparable to those for other rodents with conventional eyes. If selection has been relaxed due to blindness, but the eye still functions in some less constrained way, then an intermediate rate of change might be observed.
The eye of
S. ehrenbergi
still builds a lens (though the shape is irregular and cannot focus an image), and the lens includes a protein, called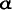 A-crystallin. The gene for this protein has recently been sequenced and compared with the corresponding gene in nine other rodents with normal vision (see article by W. Hendriks, J. Leunissen, E. Nevo, H. Bloemendal, and W. W. de Jong in bibliography).
A-crystallin. The gene for this protein has recently been sequenced and compared with the corresponding gene in nine other rodents with normal vision (see article by W. Hendriks, J. Leunissen, E. Nevo, H. Bloemendal, and W. W. de Jong in bibliography).
Hendriks and colleagues obtained the most interesting of possible results from their study. The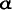 A-crystallin gene is changing much faster in blind
A-crystallin gene is changing much faster in blind
Spalax
than in other rodents with vision, as relaxation of selection due to loss of primary function would suggest. The protein coded by the
Spalax
gene, for example, has undergone nine amino acid replacements (of 173 possible changes), compared with the ancestral state for its group (the murine rodents, including rats, mice, and hamsters). All other murines in the study (rat, mouse, hamster, and gerbil) have identical sequences with no change at all from the ancestral state. The average tempo of change in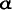 A-crystallin among vertebrates as a whole has been measured at about 3 amino acid replacements per 100 positions per 100 million years.
A-crystallin among vertebrates as a whole has been measured at about 3 amino acid replacements per 100 positions per 100 million years.
Spalax
is changing more than four times as fast, at about 13 percent per 100 million years. (Nine changes in 173 positions is 5.2 percent; but the
Spalax
lineage is only 40 million years old—and 5.2 percent in 40 million corresponds to 13 percent in 100 million years.) Moreover,
Spalax
has changed four amino acids at positions that are absolutely constant in all other vertebrates studied—seventy-two species ranging from dogfish sharks to humans.
“These findings,” Hendriks and colleagues conclude, “all clearly indicate an increased tolerance for change in the primary structure of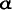 A-crystallin in this blind animal.” So far so good. But the increased tempo of change in
A-crystallin in this blind animal.” So far so good. But the increased tempo of change in
Spalax
, though marked, still reaches only about 20 percent of the characteristic rate for pseudogenes, our best standard for the maximal, truly neutral pace of evolution. Thus,
Spalax
must still be doing enough with its eyes to damp the rate of change below the maximum for neutrality. Simple models of randomness have taught us something interesting and important by setting a testable standard, approached but not met in this case, and acting as a primary criterion for judgment.
What then is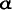 A-crystallin doing for
A-crystallin doing for
Spalax?
What can a rudimentary and irregular lens, buried under skin and hair, accomplish? We do not know, but the established intermediate rate of change leads us to ask the right questions in our search for resolution.
Spalax
is blind, but this rodent still responds to changes in photoperiod (differing lengths of daylight and darkness)—and apparently through direct influence of light regimes themselves, not by an indirect consequence that a blind animal might easily recognize (increase in temperature due to more daylight hours, for example). A. Haim, G. Heth, H. Pratt, and E. Nevo (see bibliography) showed that
Spalax
would increase its tolerance for cold weather when exposed to a winterlike light regime of eight light followed by sixteen dark hours. These mole rats were kept at the relatively warm temperature of 22°C, and were therefore not adjusting to winter based on clues provided by temperature. Animals exposed to twelve light and twelve dark hours at the same temperature did not improve their thermoregulation as well. Interestingly, animals exposed to summerlike light regimes (sixteen light and eight dark), but at colder temperatures of 17°C, actually
decreased
their cold-weather tolerance. Thus, even though blind,
Spalax
is apparently using light, not temperature, as a guide for adjusting physiology to the cycle of seasons.
Hendriks and colleagues suggest a possible explanation, not yet tested. We know that many vertebrates respond to changes in photoperiod by secreting a hormone called melatonin in the pineal gland. The pineal responds to light on the basis of photic information transmitted via the retina.
Spalax
forms a retina in its rudimentary eye, yet how can the retina, which perceives no light in this blind mammal, act in concert with the pineal gland? But Hendriks and colleagues note that the retina can also secrete melatonin itself—and that the retina of
Spalax
includes the secreting layer. Perhaps the retina of
Spalax
is still functional as a source of melatonin or as a trigger of the pineal by some mechanism still unknown. (I leave aside the fascinating, and completely unresolved, issue of how a blind animal can respond, as
Spalax
clearly does, to seasonal changes in photoperiod.)
If we accept the possibility that
Spalax
may need and use its retina (in some nonvisual way) for adaptation to changing seasons, then a potential function for the lens, and for the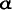 A-crystallin protein, may be sought in developmental pathways, not in direct utility. The lens cannot work in vision, and
A-crystallin protein, may be sought in developmental pathways, not in direct utility. The lens cannot work in vision, and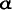 A-crystallin focuses no image, but the retina does not form in isolation and can only be generated as part of a normal embryological pathway that includes the prior differentiation of other structures. The formation of a lens vesicle may be a prerequisite to the construction of a retina—and a functioning retina may therefore require a lens, even if the lens will be used for nothing on its own.
A-crystallin focuses no image, but the retina does not form in isolation and can only be generated as part of a normal embryological pathway that includes the prior differentiation of other structures. The formation of a lens vesicle may be a prerequisite to the construction of a retina—and a functioning retina may therefore require a lens, even if the lens will be used for nothing on its own.
