Hen’s Teeth and Horse’s Toes (15 page)
Read Hen’s Teeth and Horse’s Toes Online
Authors: Stephen Jay Gould

In this sense, selfish DNA is about the worst possible name for the phenomenon, for it records the very prejudice that the new structure of explanation should be combating: an exclusive focus on bodies as evolutionary agents. When we call repetitive DNA “selfish,” we imply that it is acting for itself when it should be doing something else, namely, helping bodies in their evolutionary struggle. Likewise, we should not refer to repetitive DNA as “nonadaptive,” for although it may not be helping bodies, it is acting as its own Darwinian agent. I can’t think of a much better name in a language replete with anthropocentric terms, but how about “self-centered DNA”—without the opprobrious overtones that “selfish” inevitably contains.
Another argument against the use of selfish DNA lies in its historical source: Richard Dawkins’s book
The Selfish Gene
(1976). Dawkins argued that bodies are the wrong level of evolutionary analysis and that all evolution is nothing but a struggle among genes. Bodies are merely temporary containers for their selfish genes. Superficially, this looks like selfish DNA writ larger, hence Orgel and Crick’s decision to borrow the term. In fact, the theories of selfish genes and selfish DNA could not be more different in the structures of explanation that nurture them.
Dawkins writes as a strict Darwinian, committed to the idea that all features must be interpreted as adaptations and that all of evolution is a struggle for existence among individuals at the lowest level. He merely decided that Darwinians weren’t radical enough in reducing such higher-level reveries as “the good of the species” or “the harmony of nature” to the unrestrained struggle of organisms. The struggling items are one level lower—genes rather than bodies—and the Darwinian program of reduction can go even further than modern supporters had dared to hope.
Selfish DNA, on the other hand, gains its rationale from the antireductionistic belief that evolution works on a hierarchy of legitimate levels that cannot be collapsed to the first rung of the scale. Dawkins’s selfish genes increase in frequency because they have effects upon bodies, aiding them in their struggle for existence. Selfish DNA increases in frequency for precisely the opposite reason—because it initially has
no effect
on bodies and therefore is not suppressed at this legitimate higher level. Dawkins’s theory is an unconventional proposal to explain ordinary adaptation of bodies (see my critique in
The Panda’s Thumb
). Selfish DNA survives only because it makes no difference to bodies.
But if middle-repetitive DNA is self-centered, why does it only exist in hundreds of copies within genomes? If it can spread by transposition while other genes cannot, why does it not generate millions and billions of copies, eventually crowding everything else out? What stops it? Why is it behaving as an “intelligent” parasite (enough copies to be comfortable and powerful, but not enough to destroy the host and itself), rather than as a voracious cancer?
The potential answer to this question, proposed by both sets of authors, illustrates another interesting point about the hierarchical mode of thinking that underlies the theory of self-centered DNA. In hierarchical models, levels are not independent, walled off by impenetrable boundaries from those above and below. Levels leak and interact. Arthur Koestler, whom I do not usually praise but whose commitment to hierarchy I find admirable, chose as his metaphor for hierarchy the double-faced god Janus, standing at one level but looking for connections in both directions.
Consider different forms of selection working at levels of gene, body, and species. A transposon enters a genetic system and begins to amplify itself by replication and movement. In the process of selection among genes, it is increasing by an analog of what we would call “differential birth” in natural selection among bodies. Its increase initially produces no interaction with the level of natural selection upon bodies, and nothing suppresses its intrinsic drive to manufacture more copies.
But eventually, if its increase continues unabated, bodies must begin to notice. There is an energetic cost attached to the replication, generation after generation, of hundreds or thousands of copies of DNA sequences that do nothing for the bodies investing that energy. Bodies may not notice a few copies, but vast numbers must eventually produce a disadvantage at the good old Darwinian level of natural selection among bodies. At this point, a further increase in self-centered DNA will be suppressed because bodies carrying too many copies will suffer in natural selection, taking all their copies with them when they die or fail to reproduce. The usual level of tens to hundreds of copies may well represent a balance between inexorable increase at the level of selection among genes and eventual suppression at the next level of selection among bodies. Levels are connected by complex ties of feedback. My plea for a recognition of selection at levels other than bodies is not a negation of Darwinian theory but an attempt to enrich it.
The arguments will continue for a long time. One group of scientists notes the similarity in arrangement within chromosomes of repetitive sequences in two creatures as evolutionarily distant as the toad
Xenopus laevis
and the sea urchin
Strongylocentrotus purpuratus
. This similarity refutes self-centered DNA and points to common function, since wandering transposons, beholden only to their own level, should disperse more randomly among chromosomes. Others point out that an important transposable element in yeast and another in the fruit fly
Drosophila melanogaster
are represented in different strains of the same species by about the same number of copies, but in very different positions among chromosomes. Do the different positions represent self-centered amplification and the similarity in numbers reflect suppression at the higher level of selection upon bodies?
As with all interesting questions in natural history, the solution requires an inquiry about relative frequency, not an absolute yes or no. The logic of self-centered DNA seems sound. The question remains: how important is it? How much repetitive DNA is self-centered DNA? If the answer is “way less than one percent” because conventional selection on bodies almost always overwhelms selection among genes, then self-centered DNA is one more good and plausible idea scorned by nature. If the answer is “lots of it,” then we will need a fully articulated hierarchical theory of evolution. My own inclinations are, obviously, for hierarchy. Cartesian reductionism has been the source of science’s triumph for 300 years; but I suspect that we have reached its limits in several areas.
We have legitimate, idiosyncratic reasons for continuing our linguistic habit of identifying “individuals” with bodies, and for granting a primacy to bodies among the objects of nature. I can’t, for example, imagine any acceptable politics that does not focus upon the primacy of individual bodies—and we weep for the inhumanity of those that did not, but flourished for a time nonetheless. Nature, however, acknowledges many kinds of individuals, both great and small.
VANITY LICENSE PLATES
are the latest expression of an old conviction that distinctive conveyances reflect status or, at least, compel notice. We can build our modern machines to order, but nature has narrower limits. Horses of unusual size or color commanded great favor, but Julius Caesar ventured beyond the mere accentuation of normality in choosing his favorite mount. The historian Suetonius writes that Caesar
used to ride a remarkable horse, which had feet that were almost human, the hoofs being cleft like toes. It was born in his own stables, and as the soothsayers declared that it showed its owner would be lord of the world, he reared it with great care, and was the first to mount it; it would allow no other rider.
Normal horses represent the limit of evolutionary trends for the reduction of toes. Ancestral
Hyracotherium
(popularly, but incorrectly, known as
Eohippus
) had four toes in front and three in back, while some earlier forebear undoubtedly possessed the original mammalian complement of five on each foot. Modern horses retain but a single toe, the third of an original five. They also develop vestiges of the old second and fourth toes as short splints of bone, mounted high and inconspicuously above the hoof.
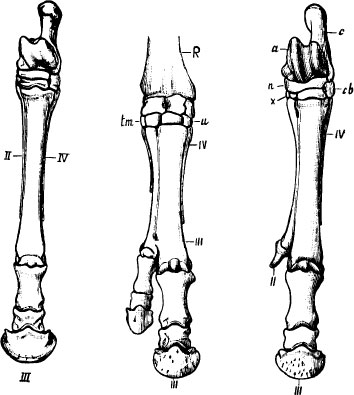
Marsh’s 1892 figures of polydactyl horses. Left, a normal horse. Note the splint remnants of side toes labeled II and IV. Middle: polydactyly by duplication. The side splints are still present and the extra toe is a duplicated third digit. Right: polydactyly by atavism. The extra toe is an enlarged side splint.
Marsh’s 1892 figure of a polydactyl horse, the “horned horse from Texas.”
Abnormal horses with extra digits have been admired and studied since Caesar’s time. O. C. Marsh, a founder of vertebrate paleontology in America, took a special interest in these aberrant animals and published a long article on “Recent polydactyle horses” in April 1892. Marsh had two major claims upon fame, one dubious—his acrimonious battles with E. D. Cope in collecting and describing vertebrate fossils from the American West—and one unambiguous—his success in deciphering the evolution of horses, the first adequate demonstration of descent provided by the fossil record of vertebrates, and an important support in Darwin’s early battles.
Marsh was puzzled and fascinated by these aberrant horses with extra toes. In most cases, the additional toe is merely a duplicate copy of the functional third digit. But Marsh found that many two- and three-toed horses had harkened back to their ancestors by developing either or both of the side splints into functional (or nearly functional) hoofed toes. (A later, and particularly thorough, German monograph of 1918 concluded that about two-thirds of horses with extra toes had simply duplicated the functional third digit, while about one-third had resuscitated an ancestral feature by developing the vestigial splints of their second or fourth toe into complete, hoofed digits.)
These apparent reversions to previous evolutionary states are called atavisms, after the Latin
atavus:
literally, great-great-great-grandfather; more generally, simply ancestor. The biological literature is studded with examples of the genre, but they have generally been treated anecdotally as mere curiosities bearing no important evolutionary message. If anything, they are surrounded with the odor of slight embarrassment, as if the progressive process of evolution did not care to be reminded so palpably of its previous imperfections. The synonyms of European colleagues express this feeling directly—“throwback” in England,
pas-en-arrière
(“backward step”) in France, and
Rückschlag
(“setback”) in Germany. When granted any general significance, atavisms have been treated as marks of constraint, as indications that an organism’s past lurks just below its present surface and can hold back its future advance.
I would suggest an opposite view—that atavisms teach an important lesson about potential results of small genetic changes, and that they suggest an unconventional approach to the problem of major transitions in evolution. In the traditional view, major transitions are a summation of the small changes that adapt populations ever more finely to their local environments. Several evolutionists, myself included, have become dissatisfied with this vision of smooth extrapolation. Must one group always evolve from another through an insensibly graded series of intermediate forms? Must evolution proceed gene by gene, each tiny change producing a correspondingly small alteration of external appearance? The fossil record rarely records smooth transitions, and it is often difficult even to imagine a function for all hypothetical intermediates between ancestors and their highly modified descendants.
One promising solution to this dilemma recognizes that certain kinds of small genetic changes may have major, discontinuous effects upon morphology. We can make no one-to-one translation between extent of genetic change and degree of alteration in external form. Genes are not attached to independent bits of the body, each responsible for building one small item. Genetic systems are arranged hierarchically; controllers and master switches often activate large blocks of genes. Small changes in the timing of action for these controllers often translate into major and discontinuous alterations of external form. Most dramatic are the so-called homeotic mutants discussed in the following essay.
The current challenge to traditional gradualistic accounts of evolutionary transitions will take root only if genetic systems contain extensive, hidden capacities for expressing small changes as large effects. Atavisms provide the most striking demonstration of this principle that I know. If genetic systems were beanbags of independent items, each responsible for building a single part of the body, then evolutionary change could only occur piece by piece. But genetic systems are integrated products of an organism’s history, and they retain extensive, latent capacities that can often be released by small changes. Horses have never lost the genetic information for producing side toes even though their ancestors settled on a single toe several million years ago. What else might their genetic system maintain, normally unexpressed, but able to serve, if activated, as a possible focus for major and rapid evolutionary change? Atavisms reflect the enormous, latent capacity of genetic systems, not primarily the constraints and limitations imposed by an organism’s past.
My latent interest in atavism was recently kindled by a report of something that has no right to exist if one of our most venerable similes expresses literal truth—hen’s teeth. On February 29, 1980 (enough of a rarity in itself), E. J. Kollar and C. Fisher reported an ingenious technique for coaxing chickens to reveal some surprising genetic flexibility retained from a distant past.
They took epithelial (outer) tissue from the first and second gill arches of a five-day-old chick embryo and combined it with mesenchyme (inner embryonic tissue) of sixteen- to eighteen-day-old mouse embryos taken from the region where first molar teeth form. A fascinating evolutionary tale lies hidden in this simple statement as well. Jaws evolved from bones supporting the anterior gills of ancestral fishes. All vertebrate embryos still develop the anterior gill arches first (as ancestral embryos did) and then transform them during development into jaws (as ancestors did not in retaining the forward gills throughout life). Thus, if the embryonic tissues of chickens still retain any capacity for forming teeth, the epithelium of the anterior gill arches is the place to look.
Kollar and Fisher took the combined embryonic tissue of mouse and chicken and grew it in what might strike readers as a bizarre and unlikely place—the anterior chambers of the eyes of adult nude mice (but where else in an animal’s body can one find an open space, filled with liquid that is not circulating?). In ordinary teeth, made by a single animal, the outer enamel layer forms from epithelial tissue and the underlying dentin and bone from mesenchyme. But mesenchyme cannot form dentin (although it can produce bone) unless it can interact directly with epithelium destined to form enamel. (In embryological jargon, epithelium is a necessary inducer, although only mesenchyme can form dentin.)
When Kollar and Fisher grafted mouse mesenchyme alone into the eyes of their experimental animals, no dentin developed, but only spongy bone—the normal product of mesenchyme when deprived of contact with enamel epithelium as an inducer. But among fifty-five combined grafts of mouse mesenchyme and chick epithelium, ten produced dentin. Thus, chick epithelium is still capable of inducing mesenchyme (from another species in another vertebrate class yet!) to form dentin.
Archaeopteryx
, the first bird, still possessed teeth, as did several fossils from the early history of birds. But no fossil bird has produced teeth during the past sixty million years, while the toothlessness of all modern birds ranks with wings and feathers as defining characters of the class. Nonetheless, although the system has not been used on its home ground for perhaps a hundred million generations, chick epithelium can still induce the formation of dentin when combined with appropriate mesenchyme (chick mesenchyme itself has probably lost the ability to form dentin, hence the toothlessness of hens and the necessity for using mice).
Kollar and Fisher then found something even more interesting. In four of their grafts, complete teeth had developed! Chick epithelium had not only induced mouse mesenchyme to form dentin; it had also been able to generate enamel matrix proteins. (Dentin must be induced by epithelium, but this epithelium cannot differentiate into enamel unless it, in turn, can interact with the very dentin it has induced. Since chick mesenchyme cannot form dentin, chick epithelium never gets the chance to show its persistent stuff in nature.)
One final point stunned me even more. Kollar and Fisher write of their best tooth: “The entire tooth structure was well formed, with root development in proper relation to the crown, but the latter did not have the typical first-molar morphology, since it lacked the cusp pattern usually present in intraocular grafts of first-molar rudiments.” In other words, the tooth looks normal, but it does not have the form of a mouse’s molar. The odd form may, of course, simply result from the peculiar interaction of two systems not meant to be joined in nature. But is it possible that we are seeing, in part, the actual form of a latent bird’s tooth—the potential structure that chick epithelium has encoded for sixty million years but has not expressed in the absence of dentin to induce it?
Kollar and Fisher’s work recalled another experiment from the opposite end of a chick, a famous story usually misreported by evolutionary biologists (once, I am embarrassed to say, by myself), as I discovered in tracking down the original source. In 1959, the French embryologist Armand Hampé reported some experiments on the development of leg bones in chick embryos. In ancestral reptiles, the tibia and fibula (the bones between your kneecap and ankle) are equal in length; the ankle region below includes a series of small bones. In
Archaeopteryx
, the first bird, tibia and fibula are still equal in length, but the ankle bones below have been reduced to two, one articulating with the tibia, the other with the fibula. In most modern birds, however, the fibula has been reduced to a splint. It never reaches the ankle region, while the two ankle bones are “engulfed” by the rapidly growing tibia and fuse with it. Thus, modern birds develop a single structure (the tibia with ankle bones fused to it and the rudimentary fibula at its side), articulating with bones of the foot below.
Hampé reasoned that the fibula might well maintain its capacity for attaining full, ancestral length, but that competition for material by the rapidly growing tibia might deprive it of any opportunity to express this potential. He therefore performed three types of experiments, all directed toward giving the fibula some relief from its imperialistic and normally victorious neighboring bone. In all cases, the fibula attained its ancestral length, equal to the tibia and reaching the ankle region below. In the first, Hampé simply grafted more embryonic tissue into the region of the growing leg bones. The tibia reached its characteristic length, but the region now had enough material “left over” for the fibula. In the second, he altered the direction of growth for tibia and fibula so that the two bones did not remain in intimate contact. In the third, he inserted a mica plate between the two bones; the developing tibia could no longer “grab” material from its less vigorous neighbor and the fibula achieved its full length.
