Hen’s Teeth and Horse’s Toes (16 page)
Read Hen’s Teeth and Horse’s Toes Online
Authors: Stephen Jay Gould

Thus, Hampé recreated an ancestral relationship between two bones by a series of simple manipulations. And this alteration engendered an even more interesting consequence. In normal chicks, the fibula begins its growth in contact with one of the small ankle bones below. But as the tibia enlarges and predominates, this contact breaks at about the fifth day of development. The fibula then retreats to form its splint, while the expanding tibia engulfs both ankle bones to form a single structure. In one case during Hampé’s manipulations, the two ankle bones remained separate and did not fuse with either tibia or fibula (while both ankle bones fused with the tibia, as usual, in the other leg of the same embryo—an untreated control allowed to develop normally). In this bird, Hampé’s simple manipulation not only produced its intended result (expression of an ancestral relationship in leg bones); it also evoked the ancestral pattern of ankle bones as well.
Hampé was able to produce these impressive atavisms by simple manipulations that amount to minor, quantitative changes in timing of development or placement of embryonic tissue. Adding more tissue doesn’t simply make a bigger part with the same proportions; it leads to differential growth of one bone (the fibula) and a change in arrangement of the entire ankle area (two ankle bones, articulating separately to tibia and fibula in some cases, rather than a single tibia with both ankle bones fused to it).
Developmental patterns of an organism’s past persist in latent form. Chicks no longer develop teeth because their own mesenchyme does not form dentin, even though their epithelium can still produce enamel and induce dentin in other animals. Chicks no longer develop separate ankle bones because their fibula no longer keeps pace with the tibia during growth, but the ankle bones develop and retain their identity when fibulas are coaxed to reach their ancestral length. An organism’s past not only constrains its future; it also provides as legacy an enormous reservoir of potential for rapid morphological change based upon small genetic alterations.
Charles Darwin constructed his theory as a two-stage process: variation to supply raw material and natural selection to impart direction. It is frequently (and incorrectly) stated that he said little about variation, embarrassed as he was by ignorance about the mechanism of heredity. Many people believe that he simply treated variation as a “black box,” something to be assumed, mentioned in passing, and then forgotten. After all, if there is always enough variation for natural selection to use, why worry about its nature and causes?
Yet Darwin was obsessed with variation. His books, considered as an ensemble, devote much more attention to variation than to natural selection, for he knew that no satisfactory theory of major evolutionary change could be constructed until the causes of variation and the empirical rules of its form and amount had been elucidated. His longest book is devoted entirely to problems of variation—the two-volume
Variation of Animals and Plants Under Domestication
(1868). Darwin felt that atavism held the key to many mysteries of variation, and he devoted an entire chapter to it, closing (as I will) with these words:
The fertilized germ of one of the higher animals…is perhaps the most wonderful object in nature…. On the doctrine of reversion [atavism]…the germ becomes a far more marvellous object, for, besides the visible changes which it undergoes, we must believe that it is crowded with invisible characters…separated by hundreds or even thousands of generations from the present time: and these characters, like those written on paper with invisible ink, lie ready to be evolved whenever the organization is disturbed by certain known or unknown conditions.
MY GRANDFATHER
, who taught me to play poker and watched the Friday night fights with me every week, once took me to one of the cruelest, yet most fascinating spectacles of decades now thankfully past—the rows of malformed people forced (by an absence of other opportunities) to display themselves to a gawking public at the Ringling Brothers sideshow.
The genteel and legitimate counterpart to such public cruelty is the vast scientific literature on deformed births—a subject dignified with its own formal name as teratology, literally, the study of monsters. Although scientists are as subject as all people to the mixture of awe, horror, and curiosity that draws people to sideshows, teratology has an important rationale beyond primal fascination.
The laws of normal growth are best formulated and understood when the causes of their exceptions can be established. The experimental method itself, a touchstone of scientific procedure, rests upon the notion that induced and controlled departures from the ordinary can lay bare the laws of order. Congenitally malformed bodies are nature’s experiments, uncontrolled by intentional human art to be sure, but sources of insight nonetheless.
The early teratologists sought to understand malformations by classifying them. In the decades before Darwin, French medical anatomists developed three categories: missing parts (
monstres par défaut
), extra parts (
monstres par excès
), and normal parts in the wrong places. The folklore of monsters had long recognized the last category in tales of anthropophagi, maneaters with eyes in their shoulders and a mouth on their breast. Shakespeare alluded both to them and to some related colleagues in
Othello
when he spoke of “The Anthropophagi and men whose heads/Do grow beneath their shoulders.”
But a classification is no more than a set of convenient pigeonholes until the causes of ordering can be specified. And here nineteenth-century teratology got becalmed in its own ignorance of heredity. The establishment of genetics in our century revived a waning interest in teratology, as early Mendelians discovered the mutational basis of several common deformities.
Geneticists had particular success with one common category in the old classification—normal parts in the wrong places. They studied their favorite animal, the fruit fly,
Drosophila melanogaster
, and found a variety of bizarre transpositions. In the first of two famous examples, the halteres (organs of balance) are transformed into wings, restoring to the aberrant fly its ancestral complement of four (normal flies, as members of the order Diptera, have two wings). In the second, legs or parts of legs replace a variety of structures in the head—antennae and parts of the mouth in particular. Mutations of this sort are called homeotic.
Not all misplacements of parts represent homeosis, and this restriction is a key to the evolutionary message I shall draw further on. William Bateson, who later invented the term genetics, defined as “homeotic” only those parts that replace an organ having the same developmental or evolutionary origin (the word comes from a Greek root for “similar”). Thus, halteres are the evolutionary descendants of wings, while insect antennae, mouthparts, and legs all differentiate from similar precursors in the embryonic segments, and all presumably evolved from an ancestor with a pair of simple and similar appendages on each adult body segment. We might refer to homeosis if a human developed a second pair of arms where his legs should be, but an extra pair of arms on the chest would not qualify.
Homeotic mutants are found on all four pairs of chromosomes in
D. melanogaster
. A 1976 review by W. J. Ouweneel includes a list that runs to three full pages. But the two most famous, best studied, and elaborate sets of homeotic mutations both reside on the right arm of the third chromosome.
The first set, called the bithorax complex and abbreviated BX-C, regulates the normal development and differentiation of the fly’s posterior body segments. The larval fly is already divided into a series of segments, initially quite similar, that will differentiate into specialized adult structures. The first five larval segments build the adult head (the first forms anterior parts of the head; the second, the eyes and antennae; and the third through fifth, the various parts of the mouth). The next three segments, T
1
, T
2
, and T
3
, form the thorax. Each will bear a pair of legs in the adult, building the normal insect complement of six. The single pair of wings will differentiate in T
2
.
The next eight segments (A
1
through A
8
) form the adult’s abdomen, while the final, or caudal, segment (A
9
and A
10
) will build the adult’s posterior end. The presence of normal BX-C genes appears to be a precondition for the ordinary development of all segments behind the second thoracic. If all the genes of BX-C are deleted from the third chromosome, all larval segments behind the second thoracic (T
2
) fail to differentiate along their normal route and seem to become second thoracic segments themselves. If the adult survived, it would be a wonder to behold, with (presumably) a pair of legs on each of its numerous posterior segments. But this deletion is, in geneticist’s jargon, “lethal,” and the fly dies while still a larva. We know that the posterior segments of such aberrant flies are slated to develop as second thoracics because incipient differentiation within the larval segments serves as a sure guide to their later fate.
The bithorax complex includes at least eight genes, all located in sequence right next to each other. Edward B. Lewis (see bibliography), the distinguished geneticist from CalTech who has spent twenty years probing the complexities of BX-C, believes that these eight genes arose as repetitions of a single ancestral gene and then evolved in different directions. Just as the entire deletion of BX-C produces the striking homeotic effect of converting all posterior segments to second thoracics, several mutations in the eight genes produce homeotic results as well. The most famous mutation, called bithorax and commandeered as a name for the entire complex, converts the third thoracic segment into a second thoracic. Thus, the adult fly develops with two second thoracics and two pairs of wings, instead of one pair and a pair of halteres behind. (It is misleading to state that halteres “turn into” wings. Rather, the entire segment normally destined to be a third thoracic, and to produce halteres, develops as a second thoracic and builds wings.) In another mutation, called bithoraxoid, the first abdominal segment develops as a third thoracic, builds a pair of legs, and produces a fly with more than the usual insect number of six.
Lewis has proposed an interesting hypothesis for the normal action of BX-C genes. He believes that they are initially repressed (turned off) in the larval fly. As the fly develops, BX-C genes are progressively derepressed (turned on). The BX-C genes act as regulators—that is, they do not build parts of the body themselves but are responsible for turning on the structural genes that do code for building blocks. Adult form reflects the amount of BX-C gene-product in an embryonic segment; the more BX-C, the more posterior in appearance the segment. Lewis then argues that BX-C genes are derepressed in sequence, from the anterior point of their action (the third thoracic segment) to the back end of the animal. When a BX-C gene turns on, its product accumulates in a given segment and, simultaneously, in all segments posterior to it. BX-C first turns on in the third thoracic, and its product accumulates in all segments from the third thoracic to the posterior end. The next BX-C gene turns on in the next posterior segment, the first abdominal, and its product accumulates in all segments from the first abdominal to the posterior end. The next gene turns on in the second abdominal, and so forth. Thus, a gradient of BX-C product forms, with lowest concentration in the second thoracic and increasing amounts in a posterior direction. The more gene product, the more posterior in appearance the form of a resultant segment.
This hypothesis is consistent with the known homeotic effects of BX-C mutations. If BX-C is deleted entirely, it supplies no gene product, and all segments behind the second thoracic differentiate as second thoracics. In a mutation with opposite effect, all the BX-C genes are turned on at the same time in all segments—and all segments affected by BX-C then differentiate as eighth abdominals.
The second outstanding set of homeotics is also named for its most famous mutation—the antennapedia complex, or ANT-C. The fine structure of this complex has recently been elucidated in a series of remarkable experiments by Thomas C. Kaufman, Ricki Lewis, Barbara Wakimoto, and Tulle Hazelrigg in Kaufman’s laboratory at the University of Indiana. (I thank Dr. Kaufman for introducing me to the literature of homeosis and for patient and lucid explanations of his own work.) The BX-C genes regulate the morphology of segmentation from the third thoracic to the posterior end; ANT-C also affects the third thoracic, but then regulates development in the five segments anterior to it (the other two thoracics and the three that produce parts of the mouth). If the entire complex is deleted, then all three thoracic segments begin to differentiate as first thoracics (while the abdominals, regulated by BX-C, develop normally). Apparently, the genes of ANT-C normally turn on in the second thoracic segment and trigger the proper development of the second and third thoracics.
Kaufman and his colleagues have found that ANT-C consists of at least seven genes, not all with known homeotic effects, lying right next to each other on the right arm of the third chromosome in
D. melanogaster
. The genes are not named for their normal effects (which, after all, just yield an ordinary fly bearing nothing special for recognition) but for their rare homeotic mutations. The first, the antennapedia gene, regulates differentiation of the second thoracic segment, and normally turns on there to accomplish its appointed function. A series of mutations has been detected at this locus, all with homeotic effects consistent with this interpretation of normal function.
One dominant mutation, antennapedia itself, has the bizarre effect (as its name implies) of producing a leg where an antenna ought to be. This wayward appendage is not any old leg, but clearly a second thoracic. The antennapedia mutation apparently works by turning on in the wrong place—the antennal segment—rather than in the second thoracic segment.
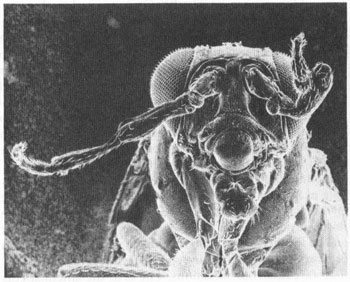
A fly with antennapedia mutation, in which legs form where the antenna should be.
SCANNING ELECTRON MICROSCOPE PHOTO BY F. R. TURNER
.
Another dominant mutation, called extra sex combs, leads to the appearance of sex combs on all three pairs of legs, not only on the first as in normal flies. This morphology is not simply the result of a gene that makes sex combs (contrary to popular belief, very few genes simply “make” individual parts without series of complex and coordinated effects). It is a homeotic mutation. All three thoracic segments differentiate as first thoracics, and the fly has, literally, three pairs of first legs with their attendant sex combs. (The entire deletion of ANT-C also causes the three thoracics to differentiate as first thoracics, but this deletion is lethal and the fly dies in its larval stage. Flies with the extra sex comb mutation do live to become adults.) The extra sex comb mutation probably operates by suppressing the normal action of its gene. Since normal action causes the second thoracic segment to differentiate properly, suppression induces all three thoracics to develop as first thoracics.
The second gene of ANT-C is named for its prominent mutation, reduced sex comb. This homeotic mutation, contrary to the effect of its neighbor extra sex comb, causes the first thoracic segment to differentiate as a second thoracic. Only first thoracic legs bear sex combs in
D. melanogaster
. The next three genes do not have known homeotic effects, and their inclusion within ANT-C is something of a puzzle. One deletes a number of embryonic segments in its most prominent mutation, another interferes with normal development of the maxilla and mandible of the mouth, while the third produces a curiously wrinkled embryo.
The sixth gene of ANT-C is named for the other famous homeotic mutant of this complex—proboscipedia, discovered in 1933 by two of the century’s most famous geneticists, Calvin Bridges and Theodosius Dobzhansky. Six mutations have been detected at this locus; most, like proboscipedia itself, produce legs where parts of the mouth should develop. The seventh and last (known) gene of ANT-C lacks homeotic effects in its mutant form and produces severe constrictions at segment boundaries in the larva. It is lethal.
Homeosis is not peculiar to fruit flies, but seems to be a general phenomenon, at least in arthropods. A set of mutations analogous (or even homologous) with the bithorax homeotics of
Drosophila
occurs in the silk moth
Bombyx
(order Lepidoptera; flies belong to the order Diptera). Two species of
Tribolium
, the flour beetle (order Coleoptera), exhibit mutations with effects that mimic the ANT-C homeotics of
Drosophila
. One set, in
Tribolium castaneum
, acts like antennapedia and produces a graded series of partial replacements of antennae by legs, ranging from tarsal claws on the eleventh antennal segment to the virtual replacement of an entire antenna with a foreleg. Another, in
T. confusum
, acts like proboscipedia and substitutes legs of the first thoracic segment for mouth structures known as labial palps. In the cockroach
Blatella germanica
, a homeotic mutant produces rudimentary wings on the first thoracic segment. No modern insect normally bears wings on its first thoracic segment, but the earliest winged fossil insects did!
