Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology
In Search of Memory: The Emergence of a New Science of Mind (31 page)

As it grows,
Aplysia
changes from a transparent, free-swimming larva that feeds on single-celled algae into a crawling, seaweed-eating juvenile slug, a small version of the adult. To achieve this radical change in body form, the larva must rest on a particular species of seaweed and be exposed to a specific chemical. No one had ever observed the metamorphosis in nature, so no one knew what the process entailed. Kriegstein observed immature
Aplysia
in the wild and noticed that they frequently rested on a particular species of seaweed. When he tested that seaweed by exposing larvae to it, he found that the larvae were transformed into juvenile slugs (figure 18–2). Most of us who were at Kriegstein’s extraordinary seminar in December 1973 will not readily forget his description of how the larvae seek out a red seaweed called
Laurencia pacifica
, rest on it, and extract from it the chemicals needed to trigger metamorphosis. When Kriegstein showed the first pictures of the tiny juvenile snail, I remember saying to myself, “Babies are always so beautiful!”
After Kriegstein’s discovery, we began to grow the seaweed and soon had all the juvenile animals we needed to culture cells of the nervous system. The next major task—how to grow individual nerve cells in culture and have them form synapses—was taken on by a former student of mine, Samuel Schacher, a cell biologist. With the help of two postdoctoral fellows, Schacher soon succeeded in culturing the individual sensory neurons, motor neurons, and interneurons involved in the gill-withdrawal reflex (figure 18–3).
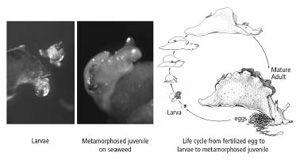
18–2
The life cycle of
Aplysia
.
Aplysia
larvae rest on a particular red seaweed (
Laurencia pacifica
) and extract from it the chemicals needed to trigger metamorphosis into a juvenile snail. (Drawing reprinted from
Cellular Basis of Behavior
, E. R. Kandel, W. H. Freeman and Company, 1976.)
We now had the elements of a learning circuit in tissue culture. This circuit enabled us to study a component of memory storage by focusing on a single sensory neuron and a single motor neuron. Our experiments showed that these isolated sensory and motor neurons form the same precise synaptic connections and exhibit the same physiological behavior in culture as they do in the intact animal. In nature, a shock to the tail activates modulatory interneurons that release serotonin, thereby strengthening the connections between sensory neurons and motor neurons. Since we already knew that these modulatory interneurons release serotonin, we found after a few experiments that we did not even need to culture them. We simply injected serotonin near the synapses between the sensory neuron and the motor neurons—that is, at the site in the intact animal where the modulatory interneurons terminate on the sensory neurons and release serotonin. One of the great pleasures of working on a biological system over a long period is seeing today’s discoveries become tomorrow’s experimental tools. Our years of study of this neural circuit, our ability to isolate the key chemical signals being transmitted between and within its cells, enabled us to use these same signals to manipulate the system and probe more deeply.
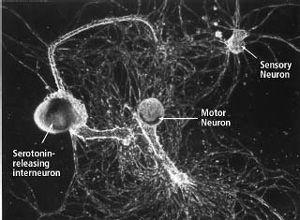
18–3 Using individual nerve cells grown in the lab to study long-term memory.
Single sensory neurons, motor neurons, and serotonin-releasing modulatory interneurons grown in culture form synapses that reproduce the simplest form of the circuit mediating and modulating the gill-withdrawal reflex. This simple learning circuit—the first available in tissue culture—made it possible to investigate the molecular biology of long-term memory. (Courtesy of Sam Schacher.)
We found that one brief pulse of serotonin strengthened the synaptic connection between the sensory and motor neuron for a few minutes by enhancing the release of glutamate from the sensory cell. As in the intact animal, this short-term enhancement of synaptic strength is a functional change: it does not require the synthesis of new proteins. In contrast, five separate pulses of serotonin, designed to simulate five shocks to the tail, strengthened the synaptic connection for days and led to the growth of new synaptic connections, an anatomical change that did involve the synthesis of new protein (figure 18–4). This showed us that we could initiate new synaptic growth in the sensory neuron in tissue culture, but we still needed to find out what proteins are important for long-term memory.
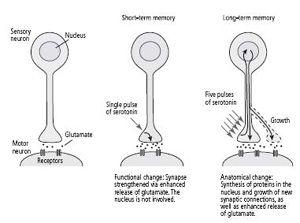
18–4
Changes underlying short- and long-term memory in a single sensory and motor neuron.
My career in neurobiology now intersected with one of the great intellectual adventures of modern biology: the unraveling of the molecular machinery for regulating genes, the coded hereditary information at the heart of every life form on earth.
THIS ADVENTURE BEGAN IN
1961
WHEN FRANÇOIS JACOB AND
Jacques Monod of the Institut Pasteur in Paris published a paper entitled “Genetic Regulatory Mechanisms in the Synthesis of Protein.” Using bacteria as a model system, they made the remarkable discovery that genes can be regulated—that is, they can be switched on and off like a water faucet.
Jacob and Monod inferred what we now know to be a fact: that even in a complex organism like a human being, almost every gene of the genome is present in every cell of the body. Every cell has in its nucleus all of the chromosomes of the organism and therefore all of the genes necessary to form the entire organism. This inference raised a serious question for biology: Why do not all genes function in the same way in every cell of the body? Jacob and Monod proposed what ultimately proved to be the case—namely, that a liver cell is a liver cell, and a brain cell is a brain cell because in each cell type only some of those genes are turned on, or expressed; all of the other genes are shut off, or repressed. Thus, each cell type contains a unique mix of proteins—a subpopulation of all the proteins available to the cell. This mix of proteins enables the cell to perform its specific biological functions.
Genes are switched on and off as needed to achieve optimal functioning of the cell. Some genes are repressed for most of the lifetime of the organism; other genes, such as those involved in the production of energy, are always expressed because the proteins they encode are essential for survival. But in every cell type some genes are expressed only at certain times, whereas others are turned on and off in response to signals from within the body or from the environment. This set of arguments caused a lightbulb to go on in my brain one night: What is learning but a set of sensory signals from the environment, with different forms of learning resulting from different types or patterns of sensory signals?
What sort of signals regulate the activity of genes? Just how are genes turned on and off? Jacob and Monod found that in bacteria, genes are switched on and off by other genes. This led them to distinguish between effector genes and regulatory genes. Effector genes encode effector proteins such as enzymes and ion channels, which mediate specific cellular functions. Regulatory genes encode proteins called gene regulatory proteins, which switch the effector genes on or off. Jacob and Monod then asked: How do the proteins of the regulatory genes act on the effector genes? They postulated that every effector gene has in its DNA not only a coding region that encodes a particular protein but also a control region, a specific site now known as the promoter. Regulatory proteins bind to the promoter of effector sites and thereby determine whether the effector genes are going to be switched on or off.
Before an effector gene can be switched on, regulatory proteins must assemble on its promoter and help to separate the two strands of DNA. One of the exposed strands is then copied into messenger RNA in a process known as transcription. Messenger RNA carries the gene’s instructions for protein synthesis from the nucleus of the cell to the cytoplasm, where structures known as ribosomes translate the messenger RNA into protein. Once the gene has been expressed, the two strands of DNA zip up again, and the gene is shut off until the next time regulatory proteins initiate transcription.
Jacob and Monod not only outlined a theory of gene regulation, they also discovered the first regulators of gene transcription. These regulators come in two forms—repressors, genes that encode the regulatory proteins that shut genes off, and as later work showed, activators, genes that encode the regulatory proteins that turn genes on. Through brilliant reasoning and insightful genetic experiments, Jacob and Monod found that when the common intestinal bacterium
E. coli
has a plentiful supply of a food source, the sugar lactose, the bacterium turns on a gene for an enzyme that breaks down lactose for consumption. When no lactose is present, the gene for this digestive enzyme is suddenly turned off. How does this occur?
The two scientists found that in the absence of lactose, the repressor gene encodes a protein that binds to the promoter of the gene for the digestive enzyme, thereby preventing the gene’s DNA from being transcribed. When they reintroduced lactose into the medium in which the bacteria were grown, the lactose moved into the cell and bound to the repressor proteins, causing them to fall off the promoter. The promoter was then free to bind proteins encoded by an activator gene. The activator proteins turn the effector gene on, resulting in production of the enzyme that metabolizes lactose.
These studies showed that
E. coli
adjusts the rate of transcription of particular genes in response to environmental cues. Later studies revealed that when a bacterium finds itself in the presence of a low concentration of glucose, it responds by synthesizing cyclic AMP, which sets off a process that enables the cell to consume an alternative sugar.
The finding that gene function can be regulated up and down in response to environmental needs by signaling molecules from outside the cell (such as different sugars) as well as from inside the cell (second messenger signals such as cyclic AMP) was revolutionary to me. It caused me to rephrase in molecular terms the question of how short-term memory is converted to long-term memory. I now asked: What is the nature of the regulatory genes that respond to a specific form of learning, that is, to cues from the environment? And how do these regulatory genes switch a short-term synaptic change that is critical to a specific short-term memory into a long-term synaptic change that is critical to a specific long-term memory?
Our studies in invertebrates, as well as several studies in vertebrates, had demonstrated that long-term memory requires the synthesis of new protein, indicating that the mechanisms of memory storage are likely to be quite similar in all animals. Moreover, Craig Bailey had made the remarkable discovery that long-term memory in
Aplysia
endures because sensory neurons grow new axon terminals that strengthen their synaptic connections with motor neurons. Yet exactly what it takes to throw the switch for any form of long-term memory remained a mystery. Does the pattern of learning that produces long-term sensitization activate certain regulatory genes, and do the proteins encoded by those genes prompt effector genes to direct the formation of new axon terminals?

