In Search of Memory: The Emergence of a New Science of Mind (41 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology

With time it became clear, however, that the James-Lange theory explains only one aspect of emotional behavior. If physiological feedback were the only controlling factor, emotions should not outlast physiological changes. Yet feelings—the thoughts and actions in response to emotion—can be sustained long after a threat has subsided. Conversely, some feelings arise much more rapidly than changes in the body. Thus there may be more to emotions than the interpretation of feedback from physiological changes in the body.
An important modification of the James-Lange view has come from the neurologist Antonio Damasio, who argues that the experience of emotion is essentially a higher order representation of the bodily reactions and that this representation can be stable and persistent. As a result of Damasio’s work, a consensus is emerging on how emotions are generated. The first step is thought to be the unconscious, implicit evaluation of a stimulus, followed by physiological responses, and finally by conscious experience that may or may not persist.
To determine directly the degree to which the initial experience of emotion is dependent upon conscious or unconscious processes, scientists had to study the internal representation of emotion with the same cellular and molecular biological tools used to study conscious and unconscious cognitive processes. They did this by combining the study of animal models with the study of people. As a result, the neural pathways of emotion have been identified with some precision in the last two decades. The unconscious component of emotion, which was identified primarily by means of animal models, involves the operation of the autonomic nervous system and the hypothalamus, which regulates it. The conscious component of emotion, studied in people, involves the evaluative functions of the cerebral cortex, which are carried out by the cingulate cortex. Central to both components is the amygdala, a group of nuclei clustered together and lying deep in the cerebral hemispheres. The amygdala is thought to coordinate the conscious experience of feeling and the bodily expression of emotion, particularly fear.
Studies of people and of rodents have found that the neural systems that store unconscious, implicit, emotionally charged memories are different from those that generate the memory of conscious, explicit feeling states. Damage to the amygdala, which is concerned with the memory of fear, disrupts the ability of an emotionally charged stimulus to elicit an emotional response. In contrast, damage to the hippocampus, which is concerned with conscious memory, interferes with the ability to remember the context in which the stimulus occurred. Thus the conscious cognitive systems give us a choice of actions, but the unconscious emotional appraisal mechanisms limit those options to a few that are appropriate to the situation. An attractive feature of this view is that it brings the study of emotion in line with studies of memory storage. The unconscious recall of emotional memory has now been shown to involve implicit memory storage, whereas conscious remembrance of the feeling state has been shown to involve explicit memory storage and therefore to require the hippocampus.
ONE STRIKING FEATURE ABOUT FEAR IS THAT IT CAN READILY
become associated with neutral stimuli through learning. Once this happens, the neutral stimuli can be powerful triggers of long-term emotional memories in people. Such learned fear is a key component of post-traumatic stress disorder, as well as social phobias, agoraphobia (fear of open spaces), and stage fright. In stage fright and other forms of anticipatory anxiety, a future event (being on stage, for example) is associated with the prospect of something going wrong (forgetting one’s lines). Post-traumatic stress disorder occurs following an extremely stressful event, such as life-threatening combat, physical torture, rape, abuse, or natural disasters. It is manifested as recurrent episodes of fear, often triggered by reminders of the initial trauma. One of the striking features of this disorder, and of learned fear in general, is that the memory of the traumatic experience remains powerful for decades and is readily reactivated by a variety of stressful circumstances. Indeed, after a single exposure to a threat, the amygdala can retain the memory of that threat throughout an organism’s entire life. How does this come about?
My entry into studies of learned fear in the mouse was in a way a natural extension of my work in
Aplysia
. In
Aplysia
, classical conditioning of fear teaches an animal to associate two stimuli: one that is neutral (a light touch on the siphon), and one that is strong enough to produce instinctive fear (a shock to the tail). Like the shock to
Aplysia
’s tail, an electric shock to the feet of a mouse elicits an instinctive fear response—withdrawal, crouching, and freezing. The neutral stimulus for mice, a simple tone, does not elicit this response. When the tone and the shock are paired repeatedly, however, the animal learns to associate the two. It learns that the tone predicts the shock. As a result, the tone by itself comes to elicit a fear response (figure 25–2).
Although the neural circuitry of learned fear in the mouse is much more complicated than that in
Aplysia
, a fair amount is known about it from the studies of Joseph LeDoux at NYU and Michael Davis, now at Emory. They found that in rodents, as in people, both innate and learned fear recruit a neural circuit focused on the amygdala. In addition, they delineated how information from the conditioned and unconditioned stimuli reaches the amygdala and how the amygdala initiates a fear response.
When a tone is paired with a shock to the feet, information about the tone and the shock are initially carried by different pathways. The tone, the conditioned stimulus, activates sensory neurons in the cochlea, the organ in the ear that receives sound. These sensory neurons send their axons to a cluster of neurons in the thalamus that is concerned with hearing. The neurons in the thalamus form two pathways: a direct pathway that goes straight to the lateral nucleus of the amygdala without ever contacting the cortex, and an indirect pathway that goes first to the auditory cortex and then to the lateral nucleus (figure 25–3). Both pathways that carry information about the tone terminate on and form synaptic connections with pyramidal neurons, the main type of nerve cell in the lateral nucleus.
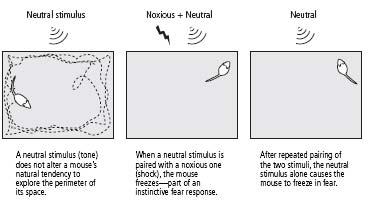
25–2
Creating learned fear in mice.
Information about pain from the unconditioned stimulus, the shock to the feet, activates pathways that terminate in a different cluster of neurons in the thalamus, one that processes painful stimuli. These neurons in the thalamus also form direct and indirect pathways to the pyramidal cells of the lateral nucleus. In this case, the indirect pathway goes through the somatosensory cortex.
The existence of separate pathways—one that goes through the cortex and one that bypasses it completely—provided direct evidence that the unconscious evaluation of a frightening stimulus precedes conscious, cortical evaluation of fear, as the James-Lange theory had predicted. By activating the fast, direct pathway that bypasses the cortex, a frightening stimulus can cause our hearts to race and our palms to sweat before we consciously realize through the slow pathway that a gun has gone off in our vicinity.
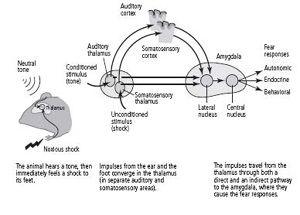
25–3
The neural pathways of learned fear.
In addition to serving as a convergence point for information about the conditioned stimulus (tone) and the unconditioned stimulus (shock), the lateral nucleus of the amygdala mobilizes adaptive responses through the connections it forms with the hypothalamus and the cingulate cortex. The hypothalamus is critical for the body’s expression of fear, triggering the fight-or-flight response (an increase in heart rate, sweating, dry mouth, and muscle tension). The cingulate cortex is concerned with conscious evaluation of fear.
HOW, THEN, DOES LEARNED FEAR IN THE MOUSE WORK? DOES IT
lead to changes in synaptic strength in the pathways affected by the conditioned stimulus, as is the case in
Aplysia?
To address this question, a number of scientists, including my colleagues and myself, studied slices of the mouse amygdala. Earlier studies had shown that both the direct and indirect pathways, when stimulated electrically at a rate similar to that used by Bliss and Lømo in the hippocampus, are strengthened through a variant of long-term potentiation. We studied this variant of long-term potentiation biochemically and found that although it differs a bit from its counterpart in the hippocampus, it is almost identical to the long-term facilitation that contributes to sensitization and classical conditioning (two forms of learned fear) in
Aplysia
. Both have a molecular signaling pathway that includes cyclic AMP, protein kinase A, and the regulatory gene CREB. These findings illustrate again that long-term facilitation and the various forms of long-term potentiation are part of a family of molecular processes capable of strengthening synaptic connections for long periods of time.
In 2002 Michael Rogan, who had previously worked with LeDoux, joined me, and we turned from studying slices of the mouse brain to studying intact animals. We examined the response of neurons in the amygdala to a tone and found, much as Rogan and LeDoux had found earlier in the rat, that learned fear increases that response (figure 25–4). This phenomenon resembled the long-term potentiation we had seen in slices of the amygdala. Our collaborator Vadim Bolshakov, at Harvard, then reasoned that if learned fear strengthens synapses in the amygdala of an intact mouse, electrical stimulation of slices of that same mouse’s amygdala should fail to produce much further synaptic strengthening. That is exactly what we found. Thus, learning acts on the same sites and in a similar manner in the amygdala of the living animal as electrical stimuli do in slices of the amygdala.
We then used a well-established behavioral test for learned fear. We placed a mouse in a large, brightly lit box. The mouse is a nocturnal animal and fears bright light, so it normally skitters along the sides of the box, making only occasional forays toward the center. This protective behavior is a compromise between the animal’s need to avoid predators and its need to explore the environment. When we sounded a tone, the mouse continued to move along the sides of the open box as if nothing had happened. But when we repeatedly followed the tone with an electric shock, the animal learned to associate the tone with the shock. When it now heard a tone, it no longer moved along the sides or entered into the center of the box; instead, it remained crouched in one corner, usually in a freezing position (figure 25–2).
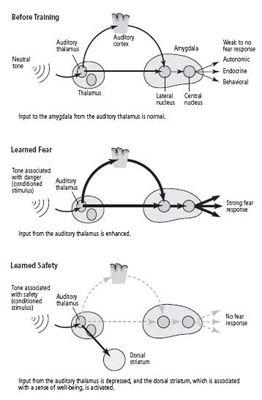
25–4 Modifying fear pathways through learning
.
WITH THIS UNDERSTANDING OF THE ANATOMY AND PHYSIOLOGY
of learned fear, we felt encouraged to explore its molecular basis. Gleb Shumyatsky, a postdoctoral fellow, and I set out to search for genes that might be expressed only in the lateral nucleus of the amygdala, the region we had been studying. We found that the pyramidal cells express a gene that encodes a peptide neurotransmitter called gastrin-releasing peptide. Pyramidal cells use this peptide as an excitatory transmitter in addition to, and in conjunction with, glutamate, releasing it from their presynaptic terminals onto target cells in the lateral nucleus. We next found that the target cells are a special population of inhibitory interneurons that contain receptors for gastrin-releasing peptide. Like all inhibitory interneurons in the lateral nucleus, these target cells release the transmitter GABA. The target cells then connect back to the pyramidal cells and, when active, release GABA to inhibit the pyramidal cells.
