Life on a Young Planet (19 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

Figure 7.1.
The Great Wall along the Kotuikan River in northern Siberia. The wall is built of flat-lying carbonate rocks deposited along the edge of the ocean some 1.5 billion years ago.
Volodya Sergeev, resplendent in army jacket and Red Sox baseball cap, has brought me here on a drizzly day in late June. Despite the weather, there is uncommon pleasure in climbing this remote ridge. Below, the taiga is shedding its winter gray for the vibrant hues of a short but intense summer. Early leaves tint the larches grass-green; roses and peonies seem to bloom everywhere; even the local foxes are shedding winter coats for summer. Owls perched on overhead branches distract us, but mosquitoes don’t; mercifully, we’re still a week away from their annual convocation. As Volodya and I pick our way up the cliff face, we debate good naturedly about the rocks beneath our feet. Each bed is scrutinized carefully. If it won’t support our arguments, it must at least support our weight.
The Bil’yakh Group—the formal name given to the Great Wall dolomites and associated rocks—continues our ascent through time. The leap from Gunflint is substantial, covering nearly a third of the 1.35-billion-year distance between those iron-rich cherts and the Cambrian. Having made the jump, what do we see? Cyanobacteria everywhere.
The Great Wall is a good place to focus on blue-greens, arguably the
most important organisms ever to appear on Earth. Cyanobacterial fossils seen earlier in cherts from Spitsbergen and the Belcher Islands hint at a general, and remarkable, feature of this group—populations preserved 750 million, 1 billion, or even 2 billion years ago are essentially indistinguishable from living forms. This is quite different from the fossil records of plants and animals, which are replete with extinct forms. Why should the evolutionary history of cyanobacteria, so much longer than that of animals, be more static, as well? This fundamental question, framed by fossils, deserves a thoughtful answer. We’ll be in a better position to attempt one after considering the illuminating treasures in Great Wall chert.
Chert nodules in the Bil’yakh Group contain lots of cyanobacterial fossils, many of them slender filaments frozen in tufts that stand tiny but erect in the rocks (
plate 4a
). More abundant, however, are spheroidal cells packed into globular colonies, like tiny cumulus clouds, along bedding surfaces (
plate 4d
).
Eoentophysalis
is the modern-looking cyanobacterium discovered by Hans Hofmann in 2.0-billion-year-old cherts of the Belcher Islands, and here, in northern Siberia, it built equally extensive mats 1.5 billion years ago. These distinctive microfossils have cells that look and divide like modern
Entophysalis
(
plate 3d
), arrayed into pigmented colonies like modern
Entophysalis
, which connect to form mats like modern
Entophysalis
, in environments like those in which
Entophysalis
thrives today. You get the picture.
Other fossils include short filaments (
plate 4b
) and tiny rods that divide by binary fission; however, Spitsbergen favorites like the stalk-forming
Polybessurus
are nowhere to be seen.
One particular Bil’yakh population deserves a closer look.
Archaeoellipsoides
is a big (in relative terms!) sausage-shaped microfossil first discovered in northern Canada by the late Bob Horodyski (
plate 4c
). These fossils are common in mid-Proterozoic cherts, but their biological interpretation remained elusive until specimens from the Great Wall provided an ID. Specifically, the sizes (up to 100 microns long) and shapes of the Siberian fossils, their lack of evidence for cell division, and the presence of germinating filaments in close proximity combined to mark
Archaeoellipsoides
as the reproductive spores of a filament-forming cyanobacterium. Modern
Anabaena
makes comparable structures.
That’s good—another fossil cyanobacterium with a close living counterpart. There is, however, special reason to be interested in
Archaeoellipsoides
.
The differentiation of specialized cell types is commonplace in animals, but rare in cyanobacteria. Only a few blue-greens are able to accomplish this trick, and they all fall on a shallow branch of the cyanobacterial tree (
figure 7.2
). Thus, if we see
Archaeoellipsoides
in 1.5billion-year-old rocks, the evolutionary diversification of cyanobacteria inferred from molecular phylogenies must have taken place earlier. Indeed, we can push a bit further back—Janine Bertrand-Sarfati, of the University of Montpellier in France, has identified
Archaeoellipsoides
in West African cherts nearly 2.1 billion years old.
The modern counterparts of
Archaeoellipsoides
actually differentiate two specialized types of cells. In addition to reproductive spores that can enter the fossil record as identifiable structures, these blue-greens form thick-walled (but not easily preserved) cells that are specialized for nitrogen fixation. Nitrogen fixation is extremely sensitive to oxygen—even modest concentrations of O
2
inhibit this process. The specialized cells in
Anabaena
and related cyanobacteria keep oxygen from diffusing into their interiors, thereby providing localized sites for nitrogen fixation in an oxygen-rich world.
The oxygen revolution discussed in
chapter 6
would, thus, have provided environmental impetus for the evolution of specialized cells in blue-greens. As
Archaeoellipsoides
fossils appear in the geological record by 2.1 billion years ago, we have a reasonable estimate of when the main branches of the cyanobacterial tree were established. By the time that microfossils first bring them into sharp focus, cyanobacteria must already have evolved much of their present-day diversity.
Paleontologists love to read stratigraphic pattern as evolutionary history, and the history suggested by Proterozoic fossils is that cyanobacteria evolved early and quickly, and then just sat there, changing little over the eons. Alternatively, however, we might imagine that while the simple shapes of cyanobacteria have remained constant through Earth history, physiology has not—something Bill Schopf long ago (but with recently restored resonance) termed the Volkswagen Syndrome. As noted in
chapter 3
, there is reason to be skeptical about this reading, because the similarities between ancient and modern cyanobacteria extend beyond form to include physiologically determined features such as life history, behavior, and environmental tolerance. Also, many features of cyanobacterial biology are conserved across the entire phylum and so must already have been present when blue-greens began to diversify.
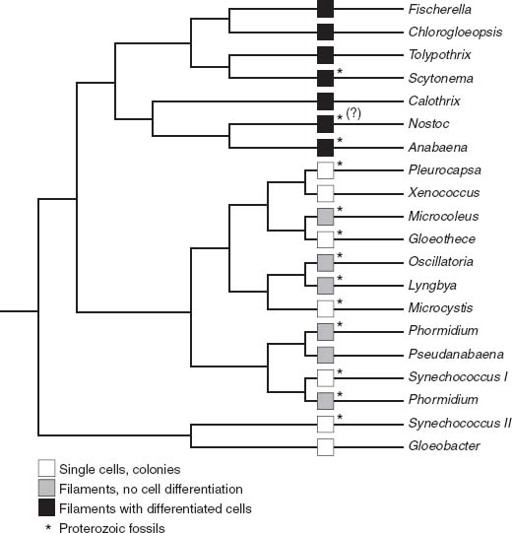
Figure 7.2.
A tree showing evolutionary relationships among living cyanobacteria. Note that cyanobacteria with specialized cells fall on a fairly late branch of the tree. This means that fossils showing cell differentiation can place an upper bound on when the tree’s major branches formed. (Phylogenetic data courtesy of Akiko Tomitani)
A more subtle problem concerns convergence. Perhaps the apparent pattern of long-term persistence in specific habitats reflects instead the
repeated molding of form and physiology by environment. If this were true, then the 2-billion-year record of
(Eo)Entophysalis
would tell us only that whenever arid tidal flats became established, cyanobacterial colonists evolved features like those of modern entophysalids. The convergence argument is hard to shake using fossils alone, but comparative biology provides reasons for doubt. If convergence rather than evolutionary relatedness explains the fit between form and environment, then we might expect morphologically similar cyanobacteria to crop up on different branches of phylogenetic trees constructed from molecular data. In fact, simple unicellular and filamentous forms do appear to have evolved repeatedly within the blue-greens—score one for convergence. In contrast, morphologically complex cyanobacteria—the forms highlighted in this book because they cannot be mistaken for other sorts of bacteria—cluster together as coherent branches in the cyanobacterial tree. For these taxa, similarity of form reflects common ancestry.
In the end, despite questions of physiology and convergence, the simplest reading of the early fossil record is probably the best one. Cyanobacteria arose long ago and early on evolved most of the molecular and morphological features seen in their living descendants.
1
Now, at last, we can return to the central riddle of cyanobacterial evolution, confident that we are framing the question correctly. Why have many blue-greens persisted so long with so little change?
Long-term stasis occurs because populations neither die off nor change. That may seem trivially obvious, but it emphasizes the fact that we have two features to explain. The general resistance of bacteria to extinction is well known. Bacteria have immense population sizes, and they can reproduce rapidly—it doesn’t matter how thoroughly you clean your teeth in the morning; the bacteria that evade your toothbrush will multiply to film your mouth by late afternoon. Bacteria also track shifting environments with ease. The air, for example, is full of bacteria,
and if you place a bowl of milk on a windowsill, it will be cheese before long. Further, bacteria are good at withstanding environmental disturbance. Although most bacterial strains grow best within a narrow habitat range, they can tolerate much more extreme conditions, at least for a short time.
Bacteria are particularly good at doing nothing. When the surrounding environment is favorable for growth, bacteria multiply rapidly, as they do in your mouth. But when ambient conditions do not favor growth, they are able to persist in a dormant stage, with little expenditure of energy. Actually, most bacteria at most times may exist in a state of metabolic torpor, ready to spring into action the moment that resources become available.
Such features explain why bacteria in general, and cyanobacteria in particular, should be persistent. But why don’t they change? Why should fossils from a 1.5-billion-year-old tidal flat look just like the cells observed in coastal mats today? The paleontological observation of long-term cyanobacterial stasis is particularly puzzling because we know that bacteria
can
evolve rapidly. New, disease-resistant strains of rice and wheat last only a decade or so before some microbe learns to take their measure. Evolved bacterial resistance to antibiotics looms as a major issue in public health.
In the laboratory, we can inoculate a culture medium with bacteria that do not grow naturally on the nutrients contained in the medium. Most cells won’t grow in their new surroundings, but a few harbor mutations that allow them to use the novel nutrients. Initially, the mutants fare poorly, but even all-thumbs growth allows survival. As mutations continue through time, natural selection hones metabolic efficiency on the new substrate. Adaptation to novel environments can easily be investigated in the course of a Ph.D. thesis or federal grant—critical timescales for laboratory biologists, but instantaneous by geological standards.
The apparent tension between rapid evolution and prolonged stasis can be resolved with help from an evolutionary metaphor introduced by Sewall Wright in 1932. In any given environment, some combinations of genes serve their owners better than others. Through time, then, natural selection will favor genetic types that grow and reproduce best. Wright thought about this interplay between genes and environment in terms
of an
adaptive landscape
. Each point on the metaphoric landscape represents a specific combination of genes; topography indicates how well each combination works in the environment. (In evolution-speak, the points are
genotypes
and the hills and valleys indicators of
fitness
.) New populations migrate toward higher ground, driven by natural selection to surmount a peak of fitness. It needn’t be the highest point on the landscape—commonly it will be the local hill most easily approached from the population’s starting point—but gene combinations that form the valley floor don’t survive for long.
Karl Niklas, a botanist at Cornell University, used a simple mathematical model to explore why some adaptive landscapes are rugged and others gentle. He found that when organisms must do many things at once, trade-offs in form and physiology enable many different genetic combinations work about equally well—the adaptive landscape consists of low rolling hills, like the Cotswolds or Pennsylvania Dutch farmland. Adaptive landscapes probably look like this for most plants and animals. In contrast, when only one functional demand must be satisfied, a single peak dominates the adaptive landscape. Bacteria can be famously single-minded.
Experiments by Richard Lenski, a microbiologist at Michigan State University, lend credence to this view. Lenski introduced a population of
E. coli
into a novel culture medium and then followed the population day by day for 10,000 generations (not quite five years). For the first 2,000 or so generations, successive generations got better and better at living in their lab environment. After that, however, improvement slowed and eventually stopped. The population evidently reached a point where additional mutations had a vanishingly small probability of improving performance.
