Life on a Young Planet (16 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

Stromatolites reinforce the theme of difference. Gunflint structures (
figure 6.2
) may look broadly like the conventional stromatolites seen in limestones, but, in detail, many of them more closely resemble sinters—laminated buildups of silica precipitated from SiO
2
-charged springs like those in Yellowstone Park. Sinters are the products of physical processes, although microorganisms can influence the details of lamination. The stromatolitic cherts of Gunflint may, therefore, reveal more about local chemistry than they do about microbial mats. In any event, Gunflint chert and iron tell us that this seaway was unusual—not like the tidal flats of Spitsbergen and, indeed, not all that similar to any habitat in the modern ocean.
Gunflint stromatolites contain huge populations of minute fossils, preserved along successive laminae within the buildups. Paleontologists debate whether these fossils record mat communities that built their stromatolitic mausoleums or bacteria that simply fell onto the accreting silica surfaces—like leaves entombed in Yellowstone sinters. My
sympathies lie with the latter camp. The dense interweaving of filaments that identifies Spitsbergen populations as mat builders is rare in Gunflint stromatolites. Far more common are layers of microfossils jumbled together without consistent orientation, like flakes of parsley sprinkled on a casserole before serving.
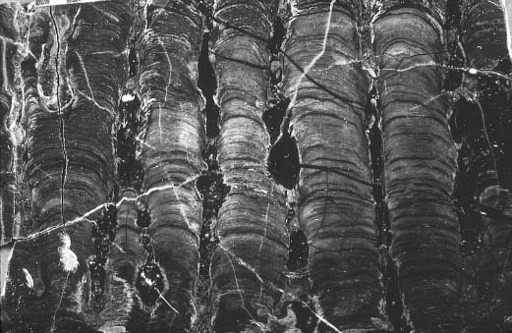
Figure 6.2.
Stromatolites in Gunflint chert. Each column is about 1 inch wide.
The most common fossils are iron-coated tubes 1–2 microns across (
plate 3a
). Appropriately named
Gunflintia minuta
, these tiny threads resemble the cyanobacterial sheaths preserved in Spitsbergen cherts, but they also compare closely with the tubular sheaths of iron-loving bacteria such as
Sphaerotilus
and
Leptothrix
(
plate 3b
), found today wherever iron-rich waters come in contact with oxygen. Other fossils support the idea that iron-loving bacteria lived in the Gunflint sea. Spherical forms a few microns in diameter differ in detail from cyanobacterial cells, but resemble coccoidal iron-loving bacteria; twisted, branching tubes resemble the modern iron-lover
Gallionella
. Rarer fossils in Gunflint stromatolites include rod-shaped cells and colonies that could be the remains of cyanobacteria, but tiny starbursts (poetically named
Eoastrion
, the “little dawn star”) strewn among the other fossils compare, once again, to bacteria that use iron and manganese in metabolism. Nonstromatolitic cherts deposited in quieter water contain starbursts in great abundance, along with small spherical cells that record some vanished plankton.
Like the fossils in Spitsbergen cherts, then, Gunflint microfossils resemble living microorganisms. But these older fossils are linked to a different set of modern counterparts—iron-metabolizing bacteria that are not much in evidence in today’s iron-starved ocean. Microfossils, thus, confirm the environmental inference drawn from iron formations. The Gunflint ocean was not like that of Spitsbergen—or the seas we know today.
Sedimentary rocks 2.1–1.8 billion years old are widely distributed, and a dozen or so are known to contain fossils. Most come from iron-rich cherts, and they closely resemble the fossils in Gunflint rocks. Gunflint’s iron-loving bacteria were not a local anomaly but rather a persistent feature of the global ocean.
Other discoveries, however, tell a different story. The most informative comes from the Belcher Islands, a cluster of low-lying islets near the
eastern shore of Hudson Bay. Here, Montreal University paleontologist Hans Hofmann collected nodules of black chert in tidal-flat carbonate beds and found fossil cyanobacteria as strikingly modern as any in Spitsbergen (plates 3c and d). Iron-loving Gunflint microbes coexisted with mat-building blue-greens.
From this and other evidence, a fuller picture of early Proterozoic life begins to emerge. Stable isotopes suggest that two billion years ago, the microbial carbon and sulfur cycles operated much as they do today. Stromatolites are abundant in carbonate rocks of this age, and many can be attributed with confidence to the activities of microbial mats. Along with the small fossils in tidal-flat cherts, they contribute to a sense of early and long-lasting
Pax cyanobacteriana
.
Where iron-rich waters welled upward from the deep to mix with the oxygenated surface ocean, Gunflint-type bacteria flourished. Soon after Gunflint time, however, fossils of this type exited the geologic record. There is no reason to believe that Gunflint-type organisms were outcompeted by expanding cyanobacteria. After all, the two types of microbial community lived side by side for many millions of years. Rather, the paleontological demise of Gunflint-type assemblages reflects loss of habitat. By 1.8 billion years ago, iron formations—the lithologic signatures of iron-rich oceans—were gone.
As we ascend through the geologic column from Gunflint toward the Kotuikan cliffs, we can anticipate discovering fossils of increasing familiarity. But first, we must look backward and ask how life changed between Warrawoona and Gunflint times. As an exercise in micropaleontology, this is difficult, because fossils that might connect the two deposits are scarce. A single fossil in 2.7-billion-year-old cherts from Australia looks tantalizingly but not unambiguously cyanobacterial, and 2.5-billion-year-old cherts from South Africa contain indifferently preserved remains that could be blue-greens, as well. Fortunately, better evidence of cyanobacterial antiquity comes from an unexpected source—biomarker molecules in 2.7-billion-year-old shales found just south of North Pole in northwestern Australia.
The biomarker evidence was unexpected for two reasons. First, until recently, cyanobacteria were not known to produce molecular signatures that are both unique and preservable. But, of course, what
organisms are “known to produce” can be quite different from what they actually make. Carefully integrating research in microbiology and organic chemistry, Roger Summons and his colleagues were able to identify distinctive lipid molecules that are synthesized in quantity only by blue-greens. Called 2-methylbacteriohopanepolyols (a name that is deeply meaningful to organic chemists and nearly incomprehensible to everyone else), these molecules are converted to molecules called 2-methylhopanes in sediments, where they can persist indefinitely as molecular fingerprints of ancient cyanobacteria.
Even accepting that cyanobacterial biomarkers can be recognized, however, their discovery in Archean shales was unanticipated. Biomarkers are destroyed by high temperatures, and conventional wisdom held that Archean sedimentary rocks were too “cooked” by metamorphism to yield well-preserved molecular fossils. Conventional wisdom isn’t entirely wrong on this point—most Archean sedimentary rocks have turbulent geological histories that make them poor targets for molecular geochemistry. But not all Archean sediments have been roasted. The trick, then, is to ignore average rocks and concentrate on the few exceptional deposits that have avoided metamorphic alteration.
Working with Roger Summons and Roger Buick, Jochen Brocks, a Ph.D. student at the University of Sydney, did just that. In 2.7-billion-year-old shales that are both exceptionally well preserved and unusually rich in organic matter, Brocks found 2-methylhopanes, confirming the Archean origins of cyanobacteria. He found other biomarkers as well, including molecules called steranes. Steranes are geologically stable molecules derived from sterols, membrane-stiffening compounds made predominantly by eukaryotes.
1
(Cholesterol is the sterol best known to most of us.) Thus, by 2.7 billion years ago (or earlier!), the Tree of Life had begun to branch, producing diverse bacteria as well as the first buds on our own, eukaryotic limb of the tree (
figure 6.3
).
Our view of evolution between 3.5 and 1.9 billion years ago is biased by a sedimentary and paleontological record that increases in abundance and quality through time. We interpret this increase as a faithful chronicle of evolutionary diversification at our peril. Nonetheless, a good argument can be made for dramatic biological changes not long before the Gunflint chert was deposited. The case, however, is not paleontological; it is geochemical.

Figure 6.3.
Molecular and isotopic biosignatures allow us to place time constraints on branch points in the Tree of Life. See text for discussion.
In
chapter 4
, I argued that iron formations provide geological evidence for oxygen scarcity in the early Archean atmosphere and oceans. By extension, the persistence of iron formations until about 1.8 billion years ago suggests that the biosphere remained oxygen poor for a very long time (
figure 6.4
). Preston Cloud championed this idea four decades ago. Slight in stature, Pres was a giant of twentieth-century paleontology who recognized long before most others that biological and environmental history are intimately intertwined. He reasoned that if oxygen levels were low when life began but high today, we should look to the geological record for evidence of environmental transformation. For Cloud, and for many who followed in his footsteps, the stratigraphic distribution of iron formations (
figure 6.5
) focused the search on early Proterozoic rocks.

Figure 6.4.
A mountain of iron. This landscape in Western Australia is carved out of a massive deposit of 2.5-billion-year-old iron formation.
Some of the most compelling evidence for oxygen scarcity on the early Earth comes from gravel and sand deposited by ancient rivers as they meandered across Archean and earliest Proterozoic coastal plains. Pyrite is common in organic-rich sediments, forming below the surface where H
2
S produced by sulfate-reducing bacteria reacts with iron dissolved in oxygen-depleted groundwaters. Crystalline pyrite also occurs in igneous and hydrothermal deposits. Despite the fact that pyrite is common in rocks, however, it almost never contributes to the sediment grains formed when rocks erode. The reason is simple. Oxygen destroys pyrite, so on the modern Earth, pyrite disappears as it is exposed and eroded.
The same is true of two other oxygen-sensitive minerals: siderite (iron carbonate, or FeCO
3
) and uraninite (uranium dioxide, or UO
2
). Neither of these minerals is found today among the eroded grains that make up sediments on coastal floodplains, but both occur with pyrite grains in river deposits older than about 2.2 billion years. During the first half of Earth history, then, pyrite, siderite, and uraninite were exposed in rock faces, stripped away by weathering and erosion, and tumbled in rivers until they came to rest in flood deposits—all without ever encountering oxygen in concentrations high enough to eliminate them.

