Life on a Young Planet (32 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll


Figure 11.2.
U-shaped burrows made by invertebrates in Cambrian beach sands. Polychaete worms make similar burrows today.
Bilaterian animals display a bewildering variety of form, but developmental and molecular data group them into three great clades (
figure 11.1
). Based on shared features of larval development, nineteenth-century zoologists united our own phylum, the Chordata, with echinoderms (starfish, sea urchins, and sea cucumbers) and a small phylum called the Hemichordata to form a supergroup dubbed the Deuterostomia. Molecular sequence comparisons support this evolutionary clustering of phyla and further divide the rest of the bilaterians (called Protostomia) into another pair of supergroups. Arthropods, nematodes, and several minor phyla link together as the Ecdysozoa—all animals in this clade form external cuticles and molt as they grow. The other great clade, infelicitously named Lophotrochozoa, includes such distinctive animals as mollusks, earthworms, brachiopods, and flatworms. Many but not all have spirally arranged cells in young embryos.
All living members of a phylum share a set of molecular and morphological
features that reflects their descent (with modification) from a common ancestor. For example, insects, crustaceans, and centipedes look very different from one another, but all are variations on a single architectural theme based on segmented bodies, jointed appendages, and external skeletons of case-hardened chitin. Arthropods, in turn, are closely related to the nematodes, or roundworms—tiny but ubiquitous animals that include the parasitic agents of hookworm disease, elephantiasis, and trichinosis. These two phyla share features that were present in
their
last common ancestor, including unusual aspects of early development, molting cuticles, and nucleotide order in gene sequences. On the other hand, arthropods and nematodes could hardly look more different—the latter are little more than tiny cylinders tapered at both ends. Their last common ancestor must, therefore, have been a relatively simple organism, not, in detail, like a nematode and
certainly
unlike any living arthropod. The obvious differences between the last common ancestor of living species within a phylum and the last common ancestor of two sister phyla highlight a fundamental point about body plan evolution. The divergence of lineages depicted by branch points in phylogenies and the evolution of complex body plans
within
lineages constitute two separate phenomena. A host of biological changes took place between the divergence of the arthropod lineage and the last common ancestor of living arthropods.
The line of descent from the last common ancestor of arthropods and nematodes to animals with recognizably arthropod body plans is littered with extinct forms—perhaps forms with segmented bodies but not chitinous skeletons or jointed legs, or, later, forms with segments and chitin, but not jointed appendages. Biologists have a particular way of speaking about these evolutionary way stations. Two concepts are so important that they qualify as one more set of Jacob Marley facts.
The
crown
group of a phylum (or class, or any other clade) includes the last common ancestor of the phylum’s living members and all its descendants (
figure 11.3
). Thus, at some moment in the latest Proterozoic or Early Cambrian, there lived a population of protoarthropods that split in two. Through time, one subpopulation gave rise to spiders, scorpions, and their relatives. The other evolved into crustaceans, insects, and
their
relatives. Some of the ur-population’s descendants became extinct, themselves—for example, the scorpionlike eurypterids that plied Paleozoic seaways. But with the origin and subsequent divergence of that founding population, the course of modern arthropod diversity was set.
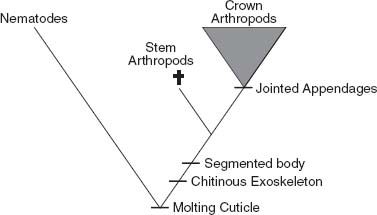
Figure 11.3.
Diagram illustrating the concepts of stem and crown groups, as exemplified by arthropod evolution. See text for discussion.
Extinct way stations that bridge the gap between crown group arthropods and the divergence of the arthropod and nematode lines are called
stem
arthropods (
figure 11.3
). To comparative biologists, steeped in the details of living animals, stem groups and last common ancestors are hypothetical constructs inferred from phylogenetic trees. Not so for paleontologists. In our world of deep time and fossils, the ancestors of living animals are real organisms that swam, crawled, or just stood still in long-vanished oceans. We don’t have to imagine them; we see them in rocks, preserved as skeletons and compressions if not actual flesh and blood. Paleontology, and paleontology alone, gives scientists access to the stem group species that illuminate the evolution of complex body plans. We
need
to understand stems and crowns to interpret the Cambrian record.
Ediacaran fossils such as
Kimberella
and
Spriggina
, which look both tantalizingly similar to and frustratingly different from modern animals, were probably stem bilaterians that lived in end-Proterozoic oceans. Not until the Cambrian, however, do we see crown group members of bilaterian phyla.
How can we understand the Cambrian emergence of a bilaterian world? The evolutionary possibilities inherent in the networks of genes that
govern development—what Berkeley developmental biologist John Gerhart and Harvard cell biologist Marc Kirschner have called “evolvability”—contribute to an explanation, as do the amplifying effects of ecology and, possibly, environmental perturbation. These command our attention, but first we need to get a grip on the most fundamental aspect of Cambrian radiation—time.
Crown group members of bilaterian phyla didn’t appear on January 1 of the Cambrian. As we saw in the Kotuikan cliffs, basal Cambrian rocks contain only a limited diversity of problematic remains, mostly small tubes made by wormlike animals and possible cnidarians. Stem groups that can be related to arthropods, or mollusks, or brachiopods appeared later, and their crown groups later yet. How much later has become clear over the past decade, as geochronologists, led by MIT’s Sam Bowring, have dated volcanic ash beds layered among fossiliferous Cambrian rocks. Tracks and trails suggest that animals with jointed legs appeared within the first 10 million years of the Cambrian, but trilobites (
plate 8
) didn’t make their entrance until the period was 20 million years old. And crown group crustaceans and chelicerates (the group that includes spiders and scorpions) may have no records older than about 511 million years, when the Cambrian had been under way for more than 30 million years.
Graham Budd and Sören Jensen, two leading paleontological Young Turks, have argued that the pattern just outlined for arthropod emergence fits other bilaterian phyla as well. For example, small caplike shells of calcium phosphate can be found in Siberian rocks formed when the Cambrian was about 13 million years old. These fossils are recognizable as brachiopods, but preserved details of shell structure show that their muscle systems and mantle tissues differed from those of any living lampshell (
figure 11.4a
). Probable crown members of the two great brachiopod lineages didn’t enter the record for another 7 to 10 million years. Then there are the helicoplacoids, baglike fossils covered by spirally arranged plates of calcium carbonate, found in rocks as old as 520–525 million years (
figure 11.4b
). Feeding structures and the microscopic details of their skeletons clearly relate helicoplacoids to the phylum Echinodermata, but these strange fossils are quite unlike any echinoderm living today. Crown group echinoderms appear only later in the Cambrian, or even in the Ordovician, depending on how one interprets a handful of mid-Cambrian fossils. As a final example, consider the great phylum Mollusca. The Ediacaran fossil
Kimberella
may be an early way station in the evolution of mollusks, and minute spiral shells made by primitive mollusks are common in rocks formed a few million years after the beginning of the Cambrian (
figure 11.4c
). Tiny stem group clams and gastropods followed, about 15 million years into the period, but the shell beds of large clams, snails, and cephalopods familiar to all paleontologists didn’t emerge as a conspicuous feature of sedimentary rocks until much later, in the Ordovician Period.
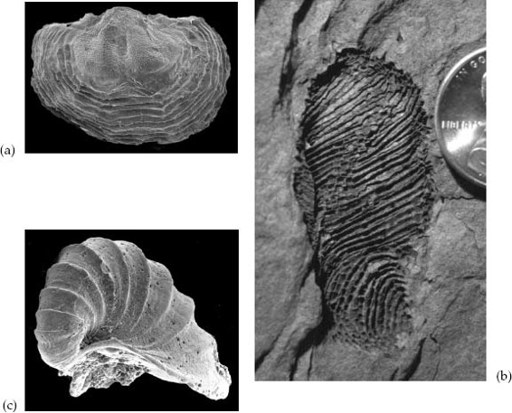
Figure 11.4.
Cambrian fossils interpreted as stem groups of bilaterian phyla or classes. (a) an Early Cambrian brachiopod. (b) A helicoplacoid echinoderm. (c) A spirally coiled mollusk from basal Cambrian rocks. See text for discussion. (Image (a) courtesy of Leonid Popov; (b) courtesy of David Bottjer and Stephen Dornbos; (c) courtesy of Stefan Bengtson)
Paleontology’s most famous fossils bring Cambrian evolution into sharp focus. The Burgess Shale is justly renowned for its remarkable store of Cambrian animals. Spectacularly detailed compressions of sponges, comb jellies, polychaete worms, priapulids, brachiopods, arthropods, and even a lancelet-like chordate tell us that by Burgess time, a great deal of body plan evolution had occurred within bilaterian phyla. But these remains share bedding surfaces with a host of mind-bending problematica that challenge paleontological understanding. The two-inch long
Opabinia
(
figure 11.5a
) had chitin-covered segments and feathery gills like any good arthropod, but it had no legs, and—worse—it sported five eyes and a grasping proboscis. The sluglike
Wiwaxia
(
figure 11.5b
), sallying forth in chain mail of chitinous scales, is equally bizarre. And so is
Anomalocaris
(
figure 11.5c
), a giant (up to two feet long!) predator distinguished by a segmented body with fanlike lobes instead of legs, but a pair of jointed appendages on the underside of its head to stuff food into its strange diaphragm-like mouth.
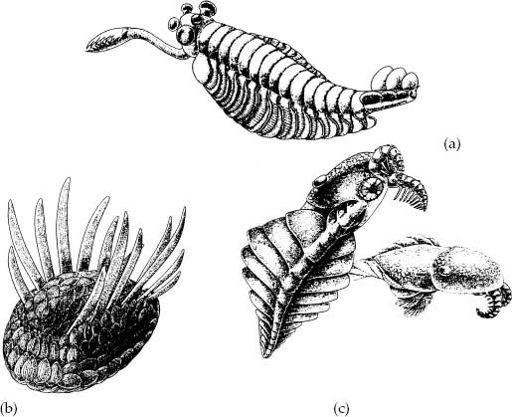
Figure 11.5.
“Weird wonders” from the Middle Cambrian Burgess Shale. (a)
Opabinia
. (b)
Wiwaxia
. (c)
Anomalocaris
. (From S. J. Gould’s
Wonderful Life
, W. W. Norton and Company, Inc., reproduced with permission)
