Read Life on a Young Planet Online
Authors: Andrew H. Knoll
Life on a Young Planet (33 page)
In
Wonderful Life
, Stephen Jay Gould focused intently on
Opabinia
,
viewing it as key to the biological interpretation of Burgess fossils.
3
Steve paid particularly careful attention to those features that separate
Opabinia
from living animals—the odd proboscis and sci-fi eyes. As a result, he assigned this fossil to an extinct phylum, like Seilacher’s vendobionts but younger (and within the fold of bilaterian animals). Steve likewise interpreted
Wiwaxia, Anomalocaris
, and other “weird wonders” as extinct body plans unlike anything seen in modern oceans.
Let us grant that some Burgess animals
are
very strange. But they aren’t completely alien. After all,
Opabinia
’s segmented body and chitinous external skeleton suggest evolutionary relationship to true arthropods.
Anomalocaris
had a segmented trunk, chitinous external skeleton,
and
jointed appendages, at least on its head. Weird or not, these fossils are stem groups that provide glimpses of how modern arthropods came to be—the concrete remains of forms mentioned earlier as hypothetical way stations in arthropod evolution.
4
And, as shown by Nick Butterfield,
Wiwaxia
scales have a microscopic structure that relates them to polychaete worms (and possibly, as well, to their evolutionary cousins, the mollusks). Even Burgess fossils once thought to represent crown groups of bilaterian phyla appear, on further investigation, to include stem taxa. For example,
Aysheaia pedunculata
, a small fossil long accepted as an early velvet worm, has a mouth and terminal appendages like those found today in a closely related phylum called the tardigrades, or water bears.
How old, then, are the stem-rich fossils of Burgess? No ash beds have been found in Burgess outcrops, but biostratigraphic correlation with other, well-dated successions indicates that these magnificent animals lived about 505 million years ago, nearly 40 million years after the start of the Cambrian Period.
5
The Burgess Shale, thus, stands as a monument
to the persistence of stem group animals as much as to the emergence of crown group bilaterian phyla. Forty million years after the Cambrian began, evolutionary way stations still played a major role in the ecology of marine environments. For many bilaterian phyla, crown group species came to dominate marine communities only some 15 to 20 million years later, during dramatically renewed diversification in the Ordovician Period.
In summary, body plans recognizable as arthropods, brachiopods, mollusks, and even chordates took shape over a 10- to 30-million-year interval during the first half of the Cambrian (
figure 11.6
). Following that, continuing evolution through the remainder of the period fashioned the coordinated sets of features seen today in the crown groups of bilaterian phyla and classes. That’s about 50 million years in all. Should we regard this time frame as uncomfortably short or uninterestingly long? Was there really a Cambrian Explosion?
Some have treated the issue as semantic—anything that plays out over tens of million of years cannot be “explosive,” and if Cambrian animals didn’t “explode,” perhaps they did nothing at all out of the ordinary. Cambrian evolution was certainly not cartoonishly fast—no surprise there. But no one who has trekked through thick successions of Proterozoic shale or limestone can doubt that Cambrian events transformed the Earth. Cambrian body plan evolution may have taken 50 million years, but those 50 million years reshaped more than
3 billion
years of biological history.
If we dismiss the notion that Cambrian evolution unfolded too slowly to be interesting, should we be worried that it all transpired too quickly? Do we need to posit some unique but poorly understood evolutionary process to explain the emergence of modern animals? I don’t think so. The Cambrian Period contains plenty of time to accomplish what the Proterozoic didn’t without invoking processes unknown to population geneticists—20 million years is a long time for organisms that produce a new generation every year or two. Explanations of Cambrian evolution must be sought elsewhere, at the place where development and ecology meet.
In tradition and approach, paleontology and molecular biology lie at opposite poles of the life sciences. Over the past decade, however, the continuum of biological inquiry has curved back on itself to form a circle,
bringing paleontologists and molecular biologists into close and mutually informative contact. We’ve already seen evidence of this in the interplay between molecular phylogeny and deep Earth history. The link is further strengthened by the common interest of paleontologists and developmental biologists in body plan evolution. For, if fossils establish the stratigraphic pattern of early animal diversification, developmental genetics shows how this evolution could have been accomplished.

Figure 11.6.
A summary of animal phylogeny and Cambrian evolution, showing first-known appearances of stem and crown group members of extant phyla, as well as animal diversity through the Cambrian Period. To aid discussion in
chapter 12
, the record of carbon isotopes across the Proterozoic-Cambrian boundary is also shown. (Reprinted with permission from A. H. Knoll and S. B. Carroll, 1999. Early animal evolution: emerging views from comparative biology and geology.
Science
284: 2129–2137. Copyright 1999 American Association for the Advancement of Science)
Let’s return to the developmental tool kit, introduced earlier. Biologists have learned a great deal about development by studying that great workhorse of laboratory genetics, the fruit fly. As in all animals, fruit fly development proceeds by both the proliferation of cells and the specification of individual cell fates. The final action in cell differentiation is the expression of genes whose protein products alter the cytoskeleton and other cytoplasmic features to form functionally distinct cells such as neurons or muscle fibers. These proteins are indeed molecular carpenters, but which carpenters get tapped to finish any given cell is determined by “upstream” genes that regulate the overall developmental patterning of the fly.
The genetic cross talk that eventually determines individual cell identities begins in the earliest stage of development, before the fertilized egg even starts to divide. Proteins manufactured from RNA messages supplied by the egg’s mother establish a concentration gradient from one end of the egg to the other. These proteins selectively promote or inhibit translation of other RNA messages, thereby defining the front and rear ends of the nascent body axis—a pattern that will be enhanced and elaborated as dividing cells begin to transcribe genes from their own nuclei. Continuing gene interactions specify ever smaller zones along the body of the developing embryo, eventually dividing it into a series of discrete segments—a hallmark of arthropod organization. Once genes have given fruit flies their segments, they start adding legs, wings,
antennae, and eyes. Genes of the
Hox
family are expressed along the developing body axis in overlapping zones, and the developmental fate of each segment reflects the combination of
Hox
proteins at work in its cells. (The eight
Hox
genes of fruit flies cluster together on two segments of a single chromosome; remarkably, the linear arrangement of these genes corresponds to their spatial order of expression in the fly.)
Once segment fate has been specified, further genes initiate structural elaborations on individual segments. A gene called
Distal-less
initiates limb development, one pair on each segment. Constrained by
Hox
gene expression and guided by additional gene products, appendages on one head segment become antennae, while those on thoracic segments develop as legs. Limb formation is suppressed completely on the fly’s abdomen.
Eyeless
, another well-studied gene, initiates eye development, while
tinman
signals the beginnings of the heart. (Genes deployed in development commonly have whimsical names.
Tinman
pays winking homage to Dorothy’s metal-clad companion in Oz, but my favorite is
sonic hedgehog
, a gene deployed in the generation of limbs, teeth, hair follicles, and other features of vertebrate bodies.)
There is much more to fruit fly development than this—enough for an entire field to develop—but the observations presented here highlight a few key points. The patterning of the fly body begins in the egg and continues by means of gene interactions that specify ever more precise portions of the developing embryo. Mutations in the terminal genes that guide formation of specific cell types tend to have individually small effects, influencing eye color, bristle number, or the like. In contrast, mutations in the regulatory genes expressed earlier in developmental networks can have dramatic consequences.
Hox
gene mutations, for example, produce monstrous, and usually lethal, body forms: walking legs may sprout where antennae were meant to go, or wings may develop on two segments instead of one. Such mutants show how strongly
Hox
genes control the basic look of the fly.
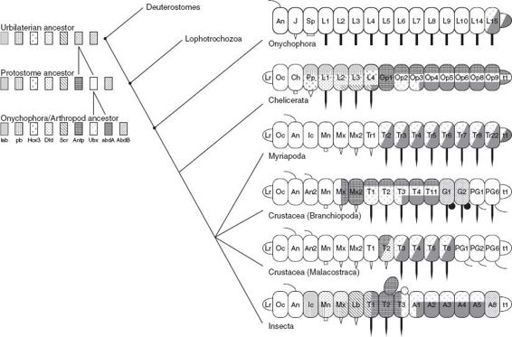
All arthropods share a common set of
Hox
genes; nonetheless, they exhibit a dazzling diversity of form. In no small part, this diversity can be related to variations in the number, identity, and morphological particulars (for example, appendage type) of the segments that form the arthropod body. Beginning with research by Michalis Averof and Michael Akam in Cambridge, England, developmental biologists have
shown that the expression patterns of
Hox
genes correlate well with these variations in segment form (
figure 11.7
). This correspondence suggests, not only that
Hox
and other regulatory genes guide arthropod development, but that
mutations
in these genes gave rise to the diversity of arthropod form seen today. Indeed, recent work in the laboratories of William McGinnis, at the University of California, San Diego, and Sean Carroll, at the University of Wisconsin, has shown how small changes in a
Hox
gene called
Ubx
governed the evolution of six-legged insects from their many-limbed crustacean ancestors.
Hox
genes in laboratory flies are beginning to put a molecular face on the Cambrian Explosion.
The story gets better. The mouse is another stalwart of laboratory research, and just as biologists have teased out the genetic circuitry of fruit fly development, they have come to understand a great deal about how mice develop. Comparison of fly and mouse genetics reveals a remarkable and unexpected correspondence. Not only do mice and flies both maintain a limited but versatile genetic tool kit for development; the two kits contain many of the same tools.
Hox
genes specify developmental fate from head to tail in mice, just as they do in flies—although a series of gene duplications have given vertebrates four sets of
Hox
genes, each equivalent to the single series found in arthropods. Genes closely related to
eyeless
and
Distal-less
induce eye and limb development in mice, as they do in flies. Even mouse hearts are initiated by a genetic homologue of
tinman
. The similarities can be astonishingly close:
eyeless-
like genes excised from a mouse can induce normal eye development in fruit flies. Of course, despite the similarity of their developmental tool kits, a mouse egg develops as a furry rodent while the fly’s becomes a tiny dive-bomber. Similar genes but different shapes—the pattern found
within
the arthropods holds
among
phyla, as well.
Spurred by the genetic commonalities of flies, mice, and the nematode
Caenorhabdites elegans
(another experimental favorite), biologists have tracked the developmental tool kit across the animal kingdom. Sponges and cnidarians maintain relatively small complements of regulatory genes, but all bilaterians share the expanded tool kit first discovered in mice and fruit flies. This means that the essential genetic prerequisites for bilaterian diversification were present in the last common ancestor of all living bilaterian animals. Based on bilaterian traces in Ediacaran rocks from the White Sea region of Russia, this ancestor must have
lived at least 555 million years ago. As the Cambrian began, then, the genetic engine of body plan evolution was already in place.