Ross & Wilson Anatomy and Physiology in Health and Illness (37 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

Blood supply
The outer layers of tissue of thick-walled blood vessels receive their blood supply via a network of blood vessels called the
vasa vasorum
. Thin-walled vessels and the endothelium of the others receive oxygen and nutrients by diffusion from the blood passing through them.
Control of blood vessel diameter
The smooth muscle in the tunica media of veins and arteries is supplied by nerves of the
autonomic nervous system
. These nerves arise from the
vasomotor centre
in the
medulla oblongata
and they change the diameter of the lumen of blood vessels, controlling the volume of blood they contain.
The blood vessels most closely regulated by this nervous mechanism are the arterioles, since they contain proportionately more smooth muscle in their walls than any other blood vessel. The walls of large arteries such as the aorta contain mainly elastic tissue and so they tend to passively expand and recoil, depending on how much blood is passing through them. Veins also respond to nerve stimulation, although they have only a little smooth muscle in their tunica media.
Blood vessel diameter and blood flow
Resistance to flow of fluids along a tube is determined by three factors: the diameter of the tube; the length of the tube; and the viscosity of the fluid involved. The most important factor determining how easily the blood flows through blood vessels is the first of these variables, that is, the diameter of the resistance vessels (the
peripheral resistance
). Peripheral resistance is a major factor in blood pressure regulation, which is further discussed on
page 87
.
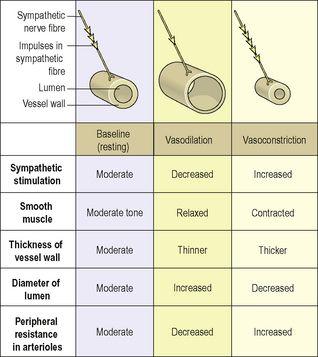
Blood vessel diameter is regulated by the smooth muscle of the tunica media, which is supplied by sympathetic nerves of the autonomic nervous system (
p. 168
). There is no parasympathetic nerve supply to most blood vessels and therefore the diameter of the vessel lumen and the tone of the smooth muscle are determined by the degree of sympathetic activity. Sympathetic activity generally constricts blood vessel smooth muscle and therefore narrows the vessel (
vasoconstriction)
, increasing the pressure inside. A degree of resting sympathetic activity maintains a constant baseline tone in the vessel wall and prevents pressure falling too low (
Fig. 5.5
). Decreased nerve stimulation relaxes the smooth muscle, thinning the vessel wall and enlarging the lumen (
vasodilation
). This results in increased blood flow under less pressure.
Figure 5.5
The relationship between sympathetic stimulation and blood vessel diameter.
Constant adjustment of blood vessel diameter helps to regulate peripheral resistance and systemic blood pressure.
Although most arterioles respond to sympathetic stimulation with vasoconstriction, the response is much less marked in some arteriolar beds, e.g. in skeletal muscle and the brain. This is important so that in a stress response, such as the flight or fight response (
p. 170
), when sympathetic activity is very high, these essential tissues are not deprived of the extra oxygen they need. The length of the vessels and viscosity of blood also contribute to peripheral resistance, but in health these are constant and are therefore not significant determinants of changes in blood flow.
Local regulation of blood flow
Since tissues’ requirements for oxygen and nutrients vary depending on their activities, it is important that blood flow is regulated locally to ensure that blood flow matches tissue needs. The ability of an organ to control its own blood flow according to need is called
autoregulation
. Some organs, including the central nervous system, liver and kidneys receive proportionately higher blood flow as a matter of course. Other tissues, such as resting skeletal muscle, receive much less, but their blood supply can increase by as much as 20-fold during heavy exercise. Other examples include blood flow through the gastrointestinal tract increasing after a meal to allow for increased activity in the tract, and adjustments to blood flow through the skin in the control of body temperature (
p. 358
). Blood flow is increased through individual organs by vasodilation of the vessels supplying it, and decreased through vasoconstriction. The main mechanisms associated with this local control of blood flow include:
•
release of metabolic waste products, e.g. CO
2
and lactic acid. An active tissue releases more wastes than a resting one, and increased levels of waste increase blood flow into the area
•
tissue temperature: a rise in metabolic activity increases tissue temperature, which in turn causes vasodilation
•
hypoxia, or lack of oxygen, stimulates vasodilation and a rise in blood flow through the affected tissue
•
release of vasodilator chemicals. Inflamed and metabolically active tissues release a number of vasodilators, which increase blood supply to the area. One important vasodilator is
nitric oxide
, which is very short lived, but which is important in opening up the larger arteries supplying an organ. Other agents include substances released in the inflammatory response, such as histamine and bradykinin (
p. 368
)
•
activity of vasoconstrictor substances. The sympathetic hormone adrenaline (epinephrine), released from the adrenal medulla, is a powerful vasoconstrictor. Others include angiotensin 2 (
p. 338
).
Capillary exchange
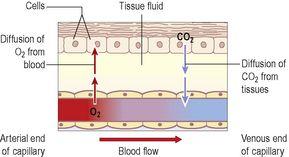
Exchange of gases
Internal respiration
(
Fig. 5.6
) is the exchange of gases between capillary blood and local body cells.
Figure 5.6
The exchange of gases in internal respiration.
Oxygen is carried from the lungs to the tissues in combination with haemoglobin as
oxyhaemoglobin
. Exchange in the tissues takes place between blood at the arterial end of the capillaries and the tissue fluid and then between the tissue fluid and the cells. Oxygen diffuses down its concentration gradient, from the oxygen-rich arterial blood, into the tissues, where oxygen levels are lower because of constant tissue consumption.
Oxyhaemoglobin is an unstable compound and breaks up (dissociates) easily to liberate oxygen. Factors that increase dissociation are discussed on
page 59
.
Carbon dioxide is one of the waste products of cell metabolism and, towards the venous end of the capillary, it diffuses into the blood down the concentration gradient. Blood transports carbon dioxide to the lungs for excretion by three different mechanisms:
•
dissolved in the water of the blood plasma – 7%
•
in chemical combination with sodium in the form of sodium bicarbonate – 70%
•
remainder in combination with haemoglobin – 23%.
Exchange of other substances
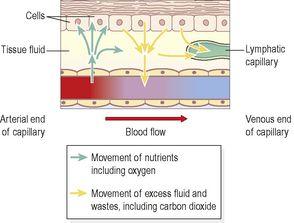
The nutrients required by the cells of the body are transported round the body in the blood plasma. In passing from the blood to the cells, the nutrients pass through the semipermeable capillary walls into the tissue fluid bathing the cells, then through the cell membrane into the cell (
Fig. 5.7
). The mechanism of the transfer of water and other substances from the blood capillaries depends mainly upon diffusion and osmosis.
Figure 5.7
Diffusion of nutrients and waste products between capillaries and cells.
Diffusion (
p. 25
)
The capillary walls consist of a single layer of epithelial cells that constitutes a semipermeable membrane, which allows small molecules to pass through into tissue fluid, and retains large molecules in the blood. Diffusible substances include dissolved oxygen and carbon dioxide, glucose, amino acids, fatty acids, glycerol, vitamins, mineral salts and water.
Osmosis (
p. 25
)
Osmotic pressure across a semipermeable membrane draws water from a dilute to a more concentrated solution to establish a state of equilibrium. The force of the osmotic pressure depends on the number of non-diffusible particles in the solutions separated by the membrane. The main substances responsible for the osmotic pressure between blood and tissue fluid are the plasma proteins, especially albumin.
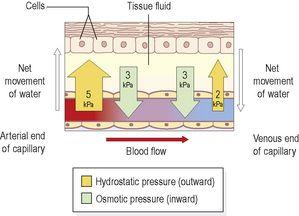
Capillary fluid dynamics
The two main forces determining overall fluid movement across the capillary wall are the
hydrostatic pressure
(blood pressure), which tends to push fluid out of the bloodstream, and the
osmotic pressure
of the blood, which tends to pull it back in, and is due mainly to the presence of plasma proteins (
Fig. 5.8
).
Figure 5.8
Effect of capillary pressures on water movement between capillaries and cells.
At
the arterial end
, the hydrostatic pressure is about 5 kPa (35 mmHg), and the opposing osmotic pressure of the blood is only 3 kPa (25 mmHg). The overall force at the arterial end of the capillary therefore drives fluid out of the capillary and into the tissue. This net loss of fluid from the bloodstream must be reclaimed in some way.
At
the venous end
of the capillary, the situation is reversed. Blood flow is slower than at the arterial end because the hydrostatic pressure drops along the capillary to only 2 kPa (15 mmHg). The osmotic pressure remains unchanged at 3 kPa (25 mmHg) and, because this now exceeds hydrostatic pressure, fluid moves back into the capillary.
This transfer of substances, including water, to the tissue spaces is a dynamic process. As blood flows slowly through the large network of capillaries from the arterial to the venous end, there is constant change. Not all the water and cell waste products return to the blood capillaries. Of the 24 litres or so of fluid that moves out of the blood across capillary walls every day, only about 21 litres returns to the bloodstream at the venous end of the capillary bed. The excess is drained away from the tissue spaces in the minute lymph capillaries which originate as blind-end tubes with walls similar to, but more permeable than, those of the blood capillaries (
Fig. 5.7
). Extra tissue fluid and some cell waste materials enter the lymph capillaries and are eventually returned to the bloodstream (
Ch. 6
).