Read The Amazing Story of Quantum Mechanics Online
Authors: James Kakalios
The Amazing Story of Quantum Mechanics (10 page)

BOOK: The Amazing Story of Quantum Mechanics
2.51Mb size Format: txt, pdf, ePub
ads
While Jon Osterman is not a real person, nor is there a wave associated with an “intrinsic field,” nor any such thing as an “intrinsic field,” for that matter, the rest of the preceding discussion about the fundamental forces of nature (that is, electromagnetism and strong and weak nuclear forces) was correct. The experiments described in Section 1 demonstrated that there
is
a wave associated with the motion of electrons and atoms, and in fact with the motion of any and all matter. The concept of a wave function, introduced by Erwin Schrödinger in his “matter-wave equation,” is the key to understanding all atomic and molecular physics. It might as well be called the “intrinsic field” for the central role it plays in the understanding of chemical bonding, by which all matter is held together.
is
a wave associated with the motion of electrons and atoms, and in fact with the motion of any and all matter. The concept of a wave function, introduced by Erwin Schrödinger in his “matter-wave equation,” is the key to understanding all atomic and molecular physics. It might as well be called the “intrinsic field” for the central role it plays in the understanding of chemical bonding, by which all matter is held together.
At the start of the twentieth century, physicists debated whether the electrical charges in an atom were spread out throughout the atom, uniformly distributed in space, or existed as concentrated pointlike negatively charged electrons and positively charged protons. A series of experiments by Ernest Rutherford, Ernest Marsden, and Hans Geiger convinced scientists by 1911 that atoms were comprised of massive, positively charged protons in a physically very small nucleus, toward which the lighter, negatively charged electrons are electrically attracted. This is the familiar “solar system” picture of the atom that you might remember from grade school. But an electrically charged solar-system atom has a big problem in stability.
The Earth is pulled toward the sun by gravity, so what keeps our planet from falling into the sun? It turns out that this is a common misconception—the Earth is falling into the sun all the time! Don’t panic—we’re not on a death spiral to a fiery end. The Earth is moving at a high velocity at nearly a right angle to an imaginary line connecting us to the sun. The gravitational pull toward the sun deflects the Earth away from its straight-line path (an object in motion will remain in motion—unless acted upon by an outside force, such as gravity from the sun). The combination of the acceleration toward the sun, and the motion at a right angle away from the sun, results in a circular trajectory (the actual orbit is an ellipse—a distorted circle). The stable orbits of the planets in our solar system are possible only through the continual falling toward the sun. The Earth maintains its orbital motion, as there is nothing to slow it down (collisions with space particles provide a very small frictional drag that we can neglect), while the conditions that led to the Earth’s original velocity about the sun are, as they say, a subject of current research.
As the force of electrical attraction between the electrons and protons in an atom is mathematically similar to the attractive force of gravity, a completely analogous argument suggests that the electrons should move in circular or elliptical orbits about the nucleus, not unlike the way the planets orbit the sun in our solar system. The main difference is that planets do not emit energy as they orbit the sun, but orbiting electrons do lose energy—in fact, quantum mechanics was developed in part to explain why all atoms
don’t
suffer a death spiral to oblivion.
don’t
suffer a death spiral to oblivion.
It was known from the days of the American Civil War that whenever an electric charge changes its direction, as in an elliptical orbit about an atomic nucleus, it emits electromagnetic radiation—that is, light. Since light carries energy, the electrons should lose energy as they orbit, and the slower they move, the less they are able to resist the attractive pull of the positively charged protons in the nucleus. In a short time (actually, less than a trillionth of a second), they should spiral into the nucleus. However, atoms form chemical bonds with other atoms, by which materials such as table salt, sand, and DNA are possible. The chemical bonds holding molecules and solids together involve interactions of the orbiting electrons among neighboring atoms, which would not be possible if the electrons were sitting on the nuclei. Something in the picture had to be wrong. If accelerated electric charges did not emit electromagnetic radiation, then radio and TV would not be possible.
21
21
An important step in reconciling this puzzle was Niels Bohr’s suggestion in 1913 that the electrons in an atom can assume only particular trajectories about the nucleus. That is, only certain planetary-like orbits are allowed. Electrons can jump from one orbit to another, but they may not follow any arbitrary path around the nucleus. An analogy: The city of Minneapolis in Minnesota contains a series of lakes that can be circumnavigated by paved paths. There are several paths encircling each lake, one intended for pedestrians, another for bicyclists, and a third for automobiles, and each pathway is separated from the others by a grassy median. Bohr’s electronic orbits were analogous to these pathways, where electrons were free to travel but were forbidden from walking on the grass, as shown in Figure 12. The closer the orbit was to the nucleus, the more tightly bound the electron would be, so that more energy would have to be supplied to remove an inner-orbit electron than would be required to remove one from an outer ring. The electron could jump from an outer pathway to an inner loop, with the emission of the appropriate amount of energy, say by emitting light. Alternatively, by absorbing just the right amount of energy, it could be promoted from an inner orbit to a higher-energy, outer orbit (provided that the path had an open, available space for the electron). Bohr proposed that, for some reason that he could not explain, the electron would not emit light during its orbit, despite the requirements of Maxwell’s equation for a charge that is constantly changing direction, but rather give off light only when moving from one orbit to another.
Bohr’s proposal that only certain discrete orbits were possible was an attempt to account for the spectrum of light emitted by different atoms. Why are neon signs red, while the light from sodium lamps has a yellow tint? Neon, sodium, and in fact all atoms have the color they do because the electrons in these atoms have a predominant absorption (and emission) at only specific colors of light. The chemical differences between neon, with ten electrons (and ten protons in its nucleus), and sodium, with eleven electrons (balanced by eleven nuclear protons), leads to the separation between the relevant orbits corresponding to light in the red and yellow portions of the electromagnetic spectrum, respectively.
One can test this quantum principle tonight at dinner. Sprinkle a little bit of salt into the table candle (not so much that you smother the flame) and you will see a distinct yellow tinge to the candle’s light. The energetic atoms in the flame will excite electronic transitions in the sodium in the salt crystals, with electrons moving from one quantum orbit to another, and when the electrons return to their original orbits, they give off yellow light. In fact, independent of sodium, the light we see from a candle results from the electrons in the hot gas atoms near the wick being excited into high-energy orbits. When the electrons return to their lower energy states, they emit photons—which is the source of the light in a flame. We cannot have a complete understanding of fire—
the
oldest technology—without an understanding of quantum mechanics.
the
oldest technology—without an understanding of quantum mechanics.
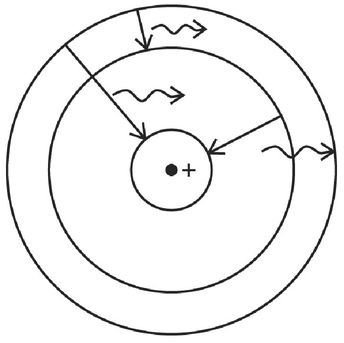
Figure 12:
Sketch of Bohr’s proposed discrete electron orbits about a positively charged nucleus. Only certain trajectories are allowed, and an electron has a different energy depending on which orbital path it is on. The electron emits or absorbs light only when moving from one orbit to another.
Sketch of Bohr’s proposed discrete electron orbits about a positively charged nucleus. Only certain trajectories are allowed, and an electron has a different energy depending on which orbital path it is on. The electron emits or absorbs light only when moving from one orbit to another.
Each element has its own unique spectrum of absorption (or emission) lines, specific wavelengths of light that correspond to allowed transitions from one electron orbit to another. Just as in the case of fingerprints or snowflakes, no two elements have exactly the same spectrum of absorption lines. By measuring the different wavelengths of the light emitted by an atom, one can identify the element or molecule. In fact, this is how the element helium, the second most common element in the universe (after hydrogen), was discovered. When absorption lines from light from the sun were studied, the line spectra for hydrogen were observed, but there was another series of lines that did not correspond to any element known on Earth. This newly detected element was named helium after the Greek god of the sun, Helios.
If you want to see what a line spectrum for a previously un-known element would look like, consider the November 1955 issue of
Strange Adventures,
a science fiction anthology comic book published by National Allied Periodicals, home of Superman and Batman. As shown in Figure 13, scientist Ken Warren uncovers the existence of a “radioactive metallic element hitherto
22
undiscovered in the entire solar system.” In order to trace the source of this new metallic element, Dr. Warren uses a scintillometer. A caption box in the story informs the readers that this is a device capable of detecting “even the smallest amount of radioactivity.” While nowadays a scintillometer refers to a device that detects small variations in the optical properties of the atmosphere, back in 1955 this was indeed the term used to measure the presence of ionizing radiation. Dr. Warren discovers that the radioactive element was brought to Earth by an alien spacecraft. This in turn leads to his capture by two would-be invaders who intend to set the entire planet aflame. Apparently if Earth could be converted into a flaming sun, then the temperature on the moon would rise to a point that would accommodate the aliens’ physiology. The fact that the sun is not actually a large planet that has been set on fire seems to have escaped these aliens, who have nevertheless managed to master interstellar flight, but it turns out to be a moot point, as Dr. Warren and his chemist colleague Hank Forrest are able to trick the aliens into abandoning their plan and leaving our solar system.
Strange Adventures,
a science fiction anthology comic book published by National Allied Periodicals, home of Superman and Batman. As shown in Figure 13, scientist Ken Warren uncovers the existence of a “radioactive metallic element hitherto
22
undiscovered in the entire solar system.” In order to trace the source of this new metallic element, Dr. Warren uses a scintillometer. A caption box in the story informs the readers that this is a device capable of detecting “even the smallest amount of radioactivity.” While nowadays a scintillometer refers to a device that detects small variations in the optical properties of the atmosphere, back in 1955 this was indeed the term used to measure the presence of ionizing radiation. Dr. Warren discovers that the radioactive element was brought to Earth by an alien spacecraft. This in turn leads to his capture by two would-be invaders who intend to set the entire planet aflame. Apparently if Earth could be converted into a flaming sun, then the temperature on the moon would rise to a point that would accommodate the aliens’ physiology. The fact that the sun is not actually a large planet that has been set on fire seems to have escaped these aliens, who have nevertheless managed to master interstellar flight, but it turns out to be a moot point, as Dr. Warren and his chemist colleague Hank Forrest are able to trick the aliens into abandoning their plan and leaving our solar system.

Figure 13:
Accurate illustration of an absorption spectrum from a 1955 DC Comics science fiction comic book. The element in question only absorbs light at very particular wavelengths, providing a unique signature as to its elemental composition. This line spectrum is a hallmark of the quantum nature of atoms.
Accurate illustration of an absorption spectrum from a 1955 DC Comics science fiction comic book. The element in question only absorbs light at very particular wavelengths, providing a unique signature as to its elemental composition. This line spectrum is a hallmark of the quantum nature of atoms.
In a sense, Bohr’s proposal that atomic line spectra arise from energy emission or absorption from electrons residing in discrete orbits managed only to reframe the mystery of atomic spectra. Now, instead of wondering why atoms absorbed or emitted light at only particular wavelengths, one could ask why only certain electronic orbits were possible about the atomic nucleus. Prince de Broglie’s matter-waves provided an answer.
Schrödinger was one of the first physicists to recognize that de Broglie’s matter-wave hypothesis could neatly solve this riddle of why the electrons did not collapse into the nucleus. For if there is indeed a wave associated with the motion of the electron, as demonstrated by Thompson’s observations reported in the pages of
Science Wonder Stories
(and the experiments shown in Figure 7), then this wave has to be a “standing wave.”
Science Wonder Stories
(and the experiments shown in Figure 7), then this wave has to be a “standing wave.”
A guitar string, clamped at both ends, as shown in Figure 14, cannot oscillate with any wavelength; only those waves are possible for which the amplitude is zero at the two fixed ends. This commonsense constraint leads to a very restricted set of possible waves that the string can support—which is why when plucked the guitar string vibrates only at certain frequencies. Wavelengths as illustrated in Figure 14b are simply not possible. This is the whole point of a musical instrument, after all, as a string that vibrated at
all
frequencies would be pretty useless for constructing harmonies. The possible waves of a clamped string, constrained by the fixed ends and unable to travel down the length of the string, are called “standing waves.”
all
frequencies would be pretty useless for constructing harmonies. The possible waves of a clamped string, constrained by the fixed ends and unable to travel down the length of the string, are called “standing waves.”


BOOK: The Amazing Story of Quantum Mechanics
2.51Mb size Format: txt, pdf, ePub
ads
Other books
The Yanti by Christopher Pike
Isobel and Emile by Alan Reed
Come Back to Me by Patrick, Coleen
Daisy's Secret by Freda Lightfoot
Dying for Love by Rita Herron
Bound by Moonlight by Nancy Gideon
Learning To Live (Zombie Overload Series) by Wright, C. M.
The Constant Gardener by John le Carre
Nothing Ever Dies: Vietnam and the Memory of War by Viet Thanh Nguyen
Wrong Room (Accidental Pleasures) by Foster, Geri
