The Amazing Story of Quantum Mechanics (7 page)
Read The Amazing Story of Quantum Mechanics Online
Authors: James Kakalios

BOOK: The Amazing Story of Quantum Mechanics
7.38Mb size Format: txt, pdf, ePub
Any solid the size of a sugar cube, such as a sugar cube, contains a little under a trillion trillion atoms. How these atoms are arranged, their chemical composition, and the nature of the connections to their neighbors determines whether the solid in question conducts electricity and is shiny (that is, reflects light), like a metal, or does not conduct electricity and is transparent to visible light, like a diamond. Consider a carbon atom, which chemically prefers to have four chemical bonds. There are many different ways a collection of carbon atoms can chemically bond to one another, and if one brings the atoms together in a haphazard, random manner, one such resulting configuration is “soot.”
12
An alternative bonding scheme would have each carbon atom located carefully in relation to its neighbors, so that all four chemical bonds for each carbon atom have their ideal strength and location. One such uniform, periodic arrangement of carbon atoms is termed “diamond.” Chemically diamond and soot are identical as they both consist of carbon atoms bonded to each other, and yet they have very different structural properties (diamond is hard, while soot is soft), electrical conduction (soot is a pretty good conductor of electricity, while diamond is an excellent insulator), optical characteristics (soot absorbs visible light, which is why it appears black, while diamond is transparent in the visible portion of the spectrum), and financial (diamond is expensive precisely in proportion to its scarcity—and don’t try to give a soot ring to your beloved
13
). If soot and diamond are identical chemically, then all of these differences must be due to the arrangements of the carbon atoms in the two substances. A deep understanding of why carbon atoms would form certain types of chemical bonds in one circumstance and very different bonds in another would not arrive until the full, formal theory of quantum mechanics was developed by Schrödinger and Heisenberg. I describe how quantum mechanics accounts for all of chemistry later on—for now we are interested in the fact that for certain solids the atoms are arranged in periodic arrays, like the oranges stacked in a grocery store display, which enables large-scale three-dimensional uniform crystalline solids.
12
An alternative bonding scheme would have each carbon atom located carefully in relation to its neighbors, so that all four chemical bonds for each carbon atom have their ideal strength and location. One such uniform, periodic arrangement of carbon atoms is termed “diamond.” Chemically diamond and soot are identical as they both consist of carbon atoms bonded to each other, and yet they have very different structural properties (diamond is hard, while soot is soft), electrical conduction (soot is a pretty good conductor of electricity, while diamond is an excellent insulator), optical characteristics (soot absorbs visible light, which is why it appears black, while diamond is transparent in the visible portion of the spectrum), and financial (diamond is expensive precisely in proportion to its scarcity—and don’t try to give a soot ring to your beloved
13
). If soot and diamond are identical chemically, then all of these differences must be due to the arrangements of the carbon atoms in the two substances. A deep understanding of why carbon atoms would form certain types of chemical bonds in one circumstance and very different bonds in another would not arrive until the full, formal theory of quantum mechanics was developed by Schrödinger and Heisenberg. I describe how quantum mechanics accounts for all of chemistry later on—for now we are interested in the fact that for certain solids the atoms are arranged in periodic arrays, like the oranges stacked in a grocery store display, which enables large-scale three-dimensional uniform crystalline solids.
These crystalline arrangements of atoms can be used as atomic-scale “oil slicks” for interference experiments, as shown in Figure 6, providing uniform layers that reflect electron beams striking them, with each layer being one atom thick, which is just the right fraction of the length of the de Broglie wavelength of our electrons. Thus, if we send in a beam of electrons traveling at the right speed, their momentum will be such that their corresponding “pilot wave” will have a de Broglie wavelength commensurate to the spacing between atomic layers in our crystal. The incoming electrons will be repelled by the electrons around each atom in the crystal—as identical electrical charges experience a repulsive force. As any given collision between the electron beam and the crystal’s electrons is random, one would expect that the intensity of scattered electrons would be fairly uniform, regardless of the direction one looks. But thanks to quantum mechanics, this is not what is seen.
Just as in the case of the light scattered from an oil slick, where all colors are present in the incident white light, but only certain colors constructively interfere, one finds that the intensity of scattered electrons is not uniform. Rather, there are regions where a high intensity of scattered electrons are found, and other regions devoid of electrons, with a pattern exactly as one would expect for interfering waves, rather than colliding particles. Figure 7 shows strikingly similar interference patterns when green light from a laser passes through a fine metal mesh and when an electron beam passes through a graphite crystal. The wavelength of green light is much longer than that of the electron beam’s de Broglie waves, so the spacing between wires in the metal screen is correspondingly larger than the separation between atoms in the carbon crystal. The intensity of scattered electrons from uniform layers of atoms in a crystal (when the electrons have a suitable momentum so that their de Broglie wavelength is equal to the spacing between crystal planes) displays an identical interference pattern as is seen when X-rays, that also have a wavelength of the same size as the atomic spacing, are reflected from the same crystal. This interference pattern holds not only for reflected electrons but also for those passing through the thin crystal, as in Thompson’s experiments summarized in the February 1930 issue of
Science Wonder Stories.
Science Wonder Stories.
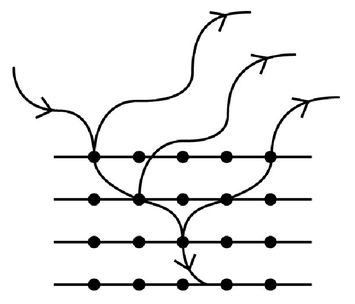
Figure 6:
Cartoon sketch of de Broglie matter waves for electrons scattering from the planes of atoms in a crystal. If the separation between atomic planes in the solid is commensurate with the de Broglie wavelength of the electrons, then interference of the scattered electrons will be observed. The intensity of electrons will be high in directions where the matter waves constructively interfere and there will be no observed electrons in directions for which destructive interference occurs.
Cartoon sketch of de Broglie matter waves for electrons scattering from the planes of atoms in a crystal. If the separation between atomic planes in the solid is commensurate with the de Broglie wavelength of the electrons, then interference of the scattered electrons will be observed. The intensity of electrons will be high in directions where the matter waves constructively interfere and there will be no observed electrons in directions for which destructive interference occurs.
As in the case of photons, described in the previous chapter, this interference effect is not a result of large numbers of electrons behaving in a collective fashion like a wave. Consider the electrons passing through the crystal in Figure 7, detected by striking a chemically coated screen that emits a flash of light whenever an electron strikes it. You’re probably familiar with this—it’s an old-style cathode-ray television tube. (Modern flat-panel liquid crystal display models work differently.) Decreasing the current of the incoming beam of electrons striking the crystal, we can arrange it so that only one electron strikes the crystal every few seconds. We would then not see a full interference pattern, but a series of individual flashes of light on the TV detector screen. The more electrons we send in, the more flashes of light. If we recorded the location of each flash and at the end of the day added them all together, instead of a uniform coverage over the screen—as would be expected if the hard-sphere electrons collided with the electrons in the crystal’s atoms, sending them randomly in all directions—we see an interference pattern as in Figure 7.
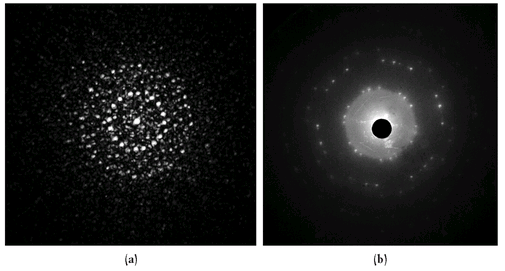
Figure 7:
Examples of light diffraction (a) and electron diffraction (b). The image on the left is obtained by passing a green laser light through a fine-mesh metal screen (not unlike a screen door) and shining the light on a wall several feet from the screen. The light scattering from the metal wires, arranged in a periodic array, leads to a symmetric constructive (bright-green spots) and destructive (dark regions) interference pattern. On the right an electron beam in a cathode ray tube passes through a graphite crystal. The momentum of the electrons is chosen so that their de Broglie wavelength is on the order of the spacing between atoms in the crystal. The atoms in the crystal scatter the electrons in a similar manner as the wire mesh does to the laser beam.
Examples of light diffraction (a) and electron diffraction (b). The image on the left is obtained by passing a green laser light through a fine-mesh metal screen (not unlike a screen door) and shining the light on a wall several feet from the screen. The light scattering from the metal wires, arranged in a periodic array, leads to a symmetric constructive (bright-green spots) and destructive (dark regions) interference pattern. On the right an electron beam in a cathode ray tube passes through a graphite crystal. The momentum of the electrons is chosen so that their de Broglie wavelength is on the order of the spacing between atoms in the crystal. The atoms in the crystal scatter the electrons in a similar manner as the wire mesh does to the laser beam.
X-rays (more about these later as well) correspond to electromagnetic waves with a wavelength roughly equal to an atomic diameter, just as for the electrons we considered in the scattering experiment. The X-rays scatter from the electrons in the crystal’s atoms, though the mechanism is a little more involved than simple electron-electron repulsion. But we can decrease the intensity of the light, so that one X-ray photon strikes the crystal every few seconds as well. Here again, a detector screen will record distinct flashes of light, and when all the flashes are added together from the scatter of many photons, the observed interference pattern is identical to that found using electrons.
14
The “dual character” symmetry between particles and waves holds for both matter and light. This, we will see, is truly the most amazing science story of the twentieth century.
14
The “dual character” symmetry between particles and waves holds for both matter and light. This, we will see, is truly the most amazing science story of the twentieth century.
CHAPTER FOUR
It’s All Done with Magnets
Everything—light and matter—has an “intrinsic angular
momentum,” or “spin,” that can have only discrete values.
momentum,” or “spin,” that can have only discrete values.
After years of unsuccessful attempts
to land a newspaper distribution deal for a comic strip featuring their creation Superman, Jerry Siegel and Joe Shuster eventually sold their story, along with the rights to the character, to the comic book publisher National Allied Periodical for $130—a nice sum in 1938, but of course a pittance compared to what the character would soon be worth. Debuting in
Action Comics
# 1 in June 1938, the Man of Tomorrow would soon be selling millions of comics per month and starring in live-action and animated movie shorts, an extremely popular radio show, and a syndicated newspaper comic strip.
to land a newspaper distribution deal for a comic strip featuring their creation Superman, Jerry Siegel and Joe Shuster eventually sold their story, along with the rights to the character, to the comic book publisher National Allied Periodical for $130—a nice sum in 1938, but of course a pittance compared to what the character would soon be worth. Debuting in
Action Comics
# 1 in June 1938, the Man of Tomorrow would soon be selling millions of comics per month and starring in live-action and animated movie shorts, an extremely popular radio show, and a syndicated newspaper comic strip.
No doubt one of the strong appeals of Siegel and Shuster’s comic-book creation is the fantasy that mild-mannered Clark Kent, dismissed and underestimated by all, is in reality the most powerful person on the planet. As Jules Feiffer argued in
The Great Comic Book Heroes,
Bruce Wayne must don his Batman costume in order to become the Caped Crusader, and Lamont Cranston his cloak, slouch hat, and red scarf to fight crime as the Shadow, but Superman is who he is. When he wakes up in the morning, he is Superman, and Clark Kent is the disguise he elects to wear. In essence, Kent is a representation of how Superman views us: weak, bumbling, inept. Superman is the iconic role model for those who feel that the world does not see their true, hidden essence.
The Great Comic Book Heroes,
Bruce Wayne must don his Batman costume in order to become the Caped Crusader, and Lamont Cranston his cloak, slouch hat, and red scarf to fight crime as the Shadow, but Superman is who he is. When he wakes up in the morning, he is Superman, and Clark Kent is the disguise he elects to wear. In essence, Kent is a representation of how Superman views us: weak, bumbling, inept. Superman is the iconic role model for those who feel that the world does not see their true, hidden essence.
One of the surprising discoveries of quantum mechanics, described in the principle at the start of this chapter, is that electrons, protons, and neutrons, the building blocks of atoms, also have a secret identity. While physicists in the 1920s knew them to be mild-mannered subatomic particles, characterized by their mass and electrical charge, it turns out that they would soon discover that the particles possessed a hidden characteristic, a superpower if you will, that is termed “spin.”

Figure 8:
In the 1960s, Dick Tracy comic strips predicted a future in which we traveled via personal flying garbage cans levitated by the power of magnetism.
In the 1960s, Dick Tracy comic strips predicted a future in which we traveled via personal flying garbage cans levitated by the power of magnetism.
It was proposed in 1925 that every fundamental particle behaves as if it is a spinning top,
15
rotating about an internal axis—and this holds not just for matter, but for photons as well. This rotation is not associated with the “orbital motion” of electrons around the nucleus in an atom (Schrödinger would eventually show that the picture of electrons circling around the positively charged nucleus, a neat analogy to the planets orbiting the sun in our solar system, is not technically accurate). This internal rotation is present even if the subatomic particles are in free space, not bound in an atom or molecule.
15
rotating about an internal axis—and this holds not just for matter, but for photons as well. This rotation is not associated with the “orbital motion” of electrons around the nucleus in an atom (Schrödinger would eventually show that the picture of electrons circling around the positively charged nucleus, a neat analogy to the planets orbiting the sun in our solar system, is not technically accurate). This internal rotation is present even if the subatomic particles are in free space, not bound in an atom or molecule.
This built-in rotation is called “spin,” for it is as if the electron is rotating about an axis passing through the particle itself—similar to a twirling ballerina. The fact that all subatomic particles have an internal rotation turns out to be pretty important. Without accounting for the spin of electrons we cannot make sense of chemistry and solid-state physics. One characteristic of all atomic particles, associated with their internal spin, is that electrons, protons, and neutrons all have an internal magnetic field that has nothing to do with the magnetic field generated by an electrical current. It is through this magnetic field that this “power,” that is, spin, first revealed itself to the world.
Other books
Charlie's Requiem Novella by A. American
Ultimate Warrior (The Fight for Creation (Book Three)) by Andrew, Saxon
Jackaby by William Ritter
The Killer's Wife by Bill Floyd
Bubble Troubles by Colleen Madden
A Canopy of Rose Leaves by Isobel Chace
MM02 - Until Morning Comes by Peggy Webb
Royal Dragon (The Bride Hunt Book 1) by Charlene Hartnady
Sleeping Angel (Ravenwood Series) by Mia James
The Knave of Hearts by Dell Shannon
