The Big Ratchet: How Humanity Thrives in the Face of Natural Crisis (10 page)
Read The Big Ratchet: How Humanity Thrives in the Face of Natural Crisis Online
Authors: Ruth DeFries

Today, only a small minority of people around the world remain in hunting and gathering societies. They are relegated to dry lands, cold climates, and inaccessible forests not fancied for farming. Our ancestors’ cumulative knowledge to manipulate the genes of plants and animals unwittingly created the most dramatic ratchet in human history. The hatchet was not far behind, and once it came down, it set in motion a new round of cumulative learning to remedy the problems that settled life had created.
S
ETTLED SOCIETIES PLACE HIGH DEMANDS
on nature and present new problems for civilization to solve. Every harvest robs the soil of fertility. Without a way to mimic the planetary machinery to replenish the soil with nitrogen, phosphorus, and about a dozen other critical nutrients that get carried away with each crop, disaster unfolds from soils depleted of life-enabling nutrients. The problem has plagued civilization from the onset of agriculture. For just as long, human ingenuity has crafted solutions of one sort or another, but it has not been an easy problem to overcome. It led Malthus to issue his dire warning at the end of the eighteenth century, and it continues to bedevil farmers today.
The oldest solution is still in play throughout the tropics. The humid, lowland forest in the far western part of the Amazon, just shy of where the Andes mountains rise to high, snow-capped peaks, is one such place. Not long ago I was fortunate to accompany a good friend and colleague near the Amazonian village where he had grown up amid a lush tangle of trees and vines. The forest provides an abundance of riches
for the local people—fruits and seeds from trees, fish from streams, and rodents and other small mammals for meat. Despite the cornucopia of different species to choose from, farming is a tough proposition in the infertile soils of the thick jungle, and people struggle to grow maize and other staple crops. One farmer proudly showed us around his homestead, where his family lives in a small and tidy wooden house. Fruit trees grace his kitchen garden. The farmer and his family live off what they can grow and perhaps sell, if there is any extra.
The Peruvian farmer hacked some sugarcane for us to chew as we walked through his neatly planted plots of maize. He guided us to some plots with tall trees growing where once he had harvested the crop. The farmer explained that he had left the trees to grow for many years. He was planning to chop them down, leave the woody debris to dry, burn it before the rains started, and replant another crop of maize in the ashes left behind. He was describing the age-old practice of slash-and-burn agriculture. Much maligned as primitive and uncivilized, slashing and burning is actually a brilliant strategy. It provides a way to break into the planetary machinery and overcome the conundrums of settled life. It has been an effective solution since the beginning of agriculture, and remains so for millions of farmers across the tropics today. The practice essentially solves the conundrum by speeding up the recycling of the nitrogen, phosphorus, and other nutrients that crops have sucked from the soil.
As vital as Earth’s recycling machinery is for our existence on this planet, it does not work in our favor when it comes to nitrogen. The protruded belly and rail-thin legs of a child suffering from the disease
kwashiorkor
—the “disease of the weaned child” that found its name in the West African nation of Ghana—exemplify the problem. The child’s
ravaged muscles and stunted growth do not result from a mere shortage of food to fill the stomach. A daily gruel of maize, cassava, rice, or some other starchy staple might ward off hunger, but it fails to provide enough protein to nourish the body. Filling as it may be, the grain-heavy diet that accompanied humanity’s transition from forager to farmer takes a toll when protein is lacking. Once a child is weaned from the rich nutrients of mother’s milk, and fed a starch-laden, protein-deficient diet, he or she starts down a path of poor health that can last a lifetime. What creativity and ingenuity might these children achieve in their lives with a bit of protein in their bowls? We will never know the answer, all because a key element of protein—nitrogen—is in the air rather than in the child’s food.
For people and all other animals, there’s only one way to get enough nitrogen into the body: eating protein in foods such as milk, meat, fish, eggs, beans, nuts, and seeds. Potatoes, rice, and other starchy foods provide energy, but carbohydrates, rather than proteins, predominate. Nitrogen nested in a protein molecule makes all the difference. Proteins make our muscles grow. They are part of every living cell. They transport oxygen through our bloodstream. Only proteins can build the hundreds of hormones and enzymes that carry out the functions of the human body, from digesting food to warding off disease. Protein is the difference between a child suffering from kwashiorkor and a healthy, growing body and mind.
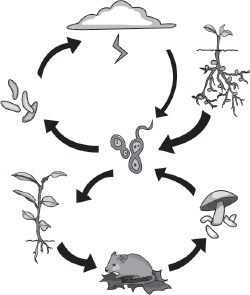
People and other animals cannot store nitrogen. Our bodies need to continually replenish the supply. Plants take up nitrogen from the soil through their roots to build their cells and tissues. We eat those plants, or we get nitrogen from eating other animals that eat plants, or from eating animals that eat other plant-eating animals. The nitrogen passes up the food chain.
On first glance, it seems like the starving child should have no problem getting enough protein to replenish nitrogen in cells and grow to be strong and healthy. After all, nitrogen is plentiful in the planetary
machine. Nitrogen gas is the most abundant of all gases in the atmosphere. For each scoop of air, about eight out of ten parts are nitrogen gas. The irony is that nitrogen is of no use to plants or animals in its atmospheric form. Nitrogen in the atmosphere is a gas, with two nitrogen atoms bound to each other. The bond is among the tightest found in nature. Unless the bond is broken apart, the nitrogen is simply inert gas. Plants can only make good use of nitrogen in a different chemical form. Most plants need nitrate, a compound of a single nitrogen atom bound to three oxygen atoms, to do the job. Unlike nitrogen gas, nitrate can dissolve in water in the spaces between soil particles, so that plants can slurp up the nitrogen through their roots.
The details of a nitrogen atom’s circular journey from air to soil, plant, animal, and back to the air only became clear in the mid-1800s. The German gentleman scientist Justus von Liebig earned his title as the father of the fertilizer industry by articulating a fundamental concept that brought nitrogen’s essential role to the fore. Lack of a single nutrient keeps a crop from thriving even if all other nutrients are present in sufficient amounts. But Liebig insisted that the limiting nutrient was phosphorus. British landowner Sir John Lawes and chemist Sir Henry Gilbert proved him wrong with experiments at the Rothamsted Experimental Station—the oldest agricultural research station in the world, which still runs to this day. They showed that crops fail when the soil lacks nitrogen. The rivalry between these men continued for decades.
How the strong bond in nitrogen gas busts apart into a form that plants can use was another quandary. Again, Liebig didn’t get it quite right. He thought plants got their nitrogen directly from the air. “Nature,” he wrote in 1840, “by means of the atmosphere, furnishes nitrogen to a plant in a quantity
sufficient for its normal growth.” Again, the Rothamsted experiments provided a key clue. Yields of clover and beans improved even without nitrogen added to the soil, but yields of other crops did not. The experiment’s outcome suggested that the legume was
able to extract nitrogen from the air. Moreover, barley, wheat, and other nitrogen-hungry crops grew better in soil where legumes had previously grown. Early Roman and Chinese farmers had known about this special property of clover and other legumes millennia before. Western science later revealed the reason for
the magical effect.
Bacteria are key to the process. Leguminous plants such as clover and beans have a key property that most plants do not. Legumes grow small nodules below the ground that are attached to their roots by fine hairs. Within these nodules lives a group of bacteria,
Rhizobium
, capable of busting apart the strong bond in nitrogen gas and transforming it to ammonia, three hydrogen atoms bound to one nitrogen atom. Other
Azotobacter
bacteria that live freely in the soil, unattached to a plant’s nodule, are capable of the same trick. So, too, are blue-green algae that live in rice paddies and other wet soils. When the bacteria die and decompose, the ammonia in their bodies enters the soil. Then other types
of bacteria further convert the ammonia to the plant-enriching nitrate. First,
Nitrosomonas
bacteria convert the ammonia to nitrite, two oxygen atoms bound to a nitrogen atom, and then
Nitrobacter
take over to convert nitrite to nitrate.
The conversion of nitrogen from its atmospheric form to another usable form in plants and animals took on the label “fixation,” after the alchemists’ term for converting a gas to a solid. Although bacteria are not the only means by which fixation occurs—the electric charge from lightning can also break the strong nitrogen bond and rainwater can carry the liberated nitrogen to the soil—bacteria, rather than lightning storms, do most of the work of fixation in nature’s nitrogen cycle. The result is fertile soil for civilization, but that’s not why the bacteria do it. None of this would take place if the process did not benefit the bacteria.
Nitrosomonas
and
Nitrobacter
both gain energy for their own growth in the process. The advantage to the rest of life is wholly incidental from their perspective.
The story of the legume’s root bacteria is more complicated still. The
Rhizobium
bacteria benefit from the plant and the plant benefits from the bacteria. The plant provides energy to the bacteria in the form of sugars produced from photosynthesis. In return, the plant receives fixed nitrogen in the form of ammonia. The symbiotic relationship between plant and bacteria is not, of course, worked out by agreement; rather, it evolved as the winning strategy for both to get what they need. When the arrangement first arose in the planetary scheme remains a mystery, as does the precise process by which the bacteria split apart nitrogen gas. Amazingly, the bacteria manage the feat under everyday conditions of temperature and pressure. Human ingenuity, as we will see in a later chapter, can simulate the process only with large inputs of energy under high temperatures and extreme pressure. What an affront to human ingenuity! Measly bacteria can solve the problem of nitrogen fixation, but great scientists could not.
Fixation is the key bottleneck in the nitrogen cycle that has plagued humanity’s attempts to keep soil fertile and nourish crops since agriculture began, but it is not the end of the story of nitrogen’s cycle in the planetary machine. Nitrogen must move back to the atmosphere; otherwise, fixation would pull nitrogen from the atmosphere and eventually permanently drain the life-enabling supply. Still other types of microbes come into play to complete the cycle. Bacteria and fungi decompose manure and the dead remains of plants and animals to cycle nitrate back into the soil. Bacteria such as
Pseudomonas
take up the baton to run the final leg. They use nitrate in their respiration, reforming the bond between two nitrogen atoms and putting nitrogen gas back into the atmosphere. The cycle is ready to start again.
Human ingenuity cannot re-create the planet’s entire machinery to recycle nitrogen, although the machinery does leave a few openings for manipulation. The slash-and-burn technique was the first solution, and others
came into play later on. But protein-enabling nitrogen robbed from the soil was not the only problem. Consider Justus von Liebig’s brilliant Law of the Minimum. Lack of a single nutrient, he said, keeps a crop from thriving, even if other nutrients are plentiful. And here Liebig was onto something: once nitrogen is sufficient, the next nutrient to be in short supply is likely to be phosphorus. The two go in lockstep. It’s like filling a hole-riddled bucket: once the lowest hole is plugged, the water rises only to the height of the next lowest hole. And on it goes until the water reaches the top of the bucket. The solution to one problem creates yet another puzzle for human ingenuity to resolve.
