The Cerebellum: Brain for an Implicit Self (36 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

Analyses of neuronal circuit suggest that the intermediate part of the cerebellar hemisphere involves three basic functional mechanisms: (1) an embedded adaptive mechanism, (2) an internal-model-based mechanism, and (3) a sensory cancellation mechanism. How we acquire skill in the execution of a novel voluntary movement can now be explained on the basis of combinations of these three control mechanisms.
In
Chapters 10
–
15
, we examined individual reflexes and voluntary limb and eye movements. Now, we are ready to consider a higher level of control of motor actions, in which a series of complex movements involving many body parts and even a tool are combined to achieve a certain goal. Here, the model-based control concepts proposed for the cerebellum in terms of forward and inverse models are extended to the cerebral cortex in terms of body schema and motor schema. Cooperation between these cerebral- and cerebellar-model-based controls seems to be the mechanism that enables us to perform complex motor actions such as piano playing and gymnastics. The understanding of these issues is still based largely on conceptual modeling, but major experimental advances might well occur in the near future.
As discussed in
Chapter 1
, “
Neuronal Circuitry: The Key to Unlocking the Brain
,” a controller of a motor action (popularly now called an “action controller”) is located in the premotor cortex. It receives instructions from the anterior cingulate gyrus and supplementary motor cortex and, in turn, acts on a controlled object, which nests the primary motor cortex and the segmental motor system including its peripheral effectors (
Figure 12C
). Anatomically, the premotor cortex is linked to the cerebellar hemisphere and dentate nucleus forming a cerebrocerebellar loop (
Figure 49
). It is also linked to the parietal association cortex (see
Figure 50
and following text). In an fMRI study on normal subjects, a sequential key-press task of increasing length and complexity was imposed, without changing the frequency, force, and number of single finger movements. This task involved activation of a subset of cortical areas, notably the contralateral ventral and dorsal premotor cortices, in addition to the bilateral superior parietal cortices, left inferior frontal gyrus/Broca’s area, right dentate nucleus, and left visual association cortex (
Haslinger et al., 2002
). The results of this study suggested the importance of premotor-cerebellar-parietal circuits for the studied movements. Another fMRI study showed that during musical performance, musically naïve control subjects displayed stronger activation than did pianists in the anterior cingulate cortex, right dorsal premotor cortex, both cerebellar hemispheres, and the right basal ganglia. Also while playing “parallel” and “mirror” bimanual movements (five fingers of two hands were paired in-phase and anti-phase, respectively), control subjects exhibited stronger signal increases than pianists within the supplementary motor cortex, bilateral cerebellar hemispheres and vermis, bilateral prefrontal cortex, left ventral premotor cortex, right anterior insula, and the right basal ganglia (
Haslinger et al., 2004
). These findings suggested an increased efficiency in musicians of neuronal processing within the cortical and subcortical systems controlling bimanual piano-playing movements. An fMRI study during cyclical hand tasks of spatiotemporal complexity and varying frequency also demonstrated that the dorsal premotor cortex and the cerebellum are critical sites for bimanual coordination. Subjects performed four such tasks of increasing complexity (unimanual left-right hand-, bimanual in-phase-, bimanual anti-phase-, and bimanual 90° out-of-phase movements) at four frequencies (0.9, 1.2, 1.5, 1.8 Hz). Activation in the supplementary motor area and superior parietal cortex were correlated mainly with increasing spatiotemporal complexity of the limb movements (
Debaerea et al., 2004
). The results are in accordance with the idea that the premotor cortex acts as the action controller for several types of highly skilled movements.
Figure 49. Wiring diagram for the D1 zone-dentate system.
The new abbreviations in this control system are DDN (dorsal portion of the dentate nucleus), PM (primary motor cortex), and VLx (subarea x of the ventrolateral thalamic nucleus). See
Figure 46
for the other abbreviations and symbols.
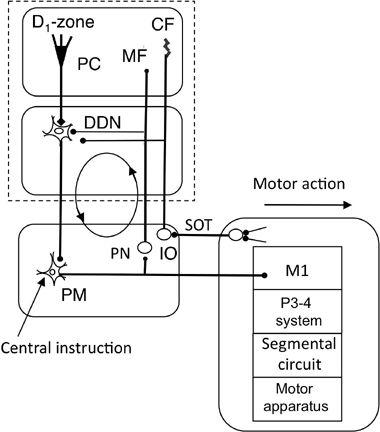
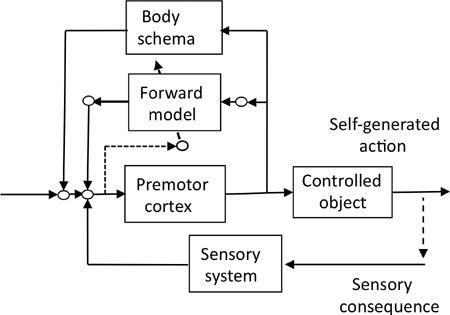
Figure 50. Model-based control of motor actions.
This block diagram shows the control system operation of the premotor cortex with the aid of a body schema and a cerebellar forward model. It is assumed that the body schema is formed during conscious effort to practice an action and that a cerebellar forward model then copies the body schema. By these means, the action can be performed automatically.
We postulated in
Chapter 1
that our cerebral neocortex acquires an “action schema” in the temporoparietal cortex, which helps the premotor cortex to control motor actions. Here, we propose that an action schema involves both “body schema” and “motor schema.” A body schema is a set of neural representations of the body and bodily functions (
Stamenov, 2005
). It may be encoded in a neuronal circuit similar to the “neuromatrix,” which was originally proposed to represent pain as a multidimensional experience produced by characteristic activity patterns of nerve impulses generated by a widely distributed neural network in the brain (
Melzack, 1990
). The related concept, motor schema, provides controllers that can be coordinated to bring about a wide variety of actions (Arbib, 2005). The body and motor schemata are heuristic concepts, which have no rigorous definition in the precise parlance of neuroscience, but their formalistic positions in the action control system resemble the cerebellar forward and inverse models proposed for voluntary motor control systems. We therefore assume that a cerebral body schema
and a cerebellar forward model are connected to the premotor cortex in parallel (
Figure 50
). Likewise a cerebral motor schema and a cerebellar inverse model may be connected in parallel.
Let us consider the steps required to learn a complex motor action. First, the premotor cortex, as an action controller, acts on the controlled object, including the primary motor cortex, to perform the motor action while relying on external sensory feedback. Second, while this process continues, the parietal cortex acquires a body schema and a motor schema by a cortical mechanism known as associative network learning (
Doya, 1999
). These schemata provide models of the learned motor action that the premotor cortex is going to perform. This initial step of learning proceeds consciously and involves the premotor and primary motor cortices and the temporo-parietal cortex. The further step of learning involves the cerebellum (see the following section).
In
Chapter 15
, “
Internal Models for Voluntary Motor Control
,” we assumed that the cerebellum uses error learning to copy the kinematics and dynamics of the controlled objects including the motor apparatus. As an analogy of this proposal, we now assume that the cerebellum copies the body schema to a forward model (
Figure 50
). An anatomical finding supporting the assumption of the forward model is that the premotor cortex and the lateral cerebellum are reciprocally linked to form a cerebrocerebellar communication loop, which parallels the loop between the primary motor cortex and the intermediate part of the cerebellum for voluntary motor control. Indeed, the premotor cortex receives a projection from the cerebellar D1-zone via the dorsal portion of the dentate nucleus and area X of the ventrolateral thalamic nucleus (
Dum and Strick, 2003
) (
Figure 51
). In turn, the premotor cortex projects to the lateral cerebellum via the pontine nucleus (
Allen et al., 1978
;
Wiesendanger et al., 1979
). The premotor cortex should thus be capable of controlling a motor action by relying on cerebellar forward models of the body schemata. On the other hand, a motor scheme may be copied in an internal inverse model by feedback error learning, which would replace the motor schema. When this late phase of learning proceeds, a motor action can be performed automatically.
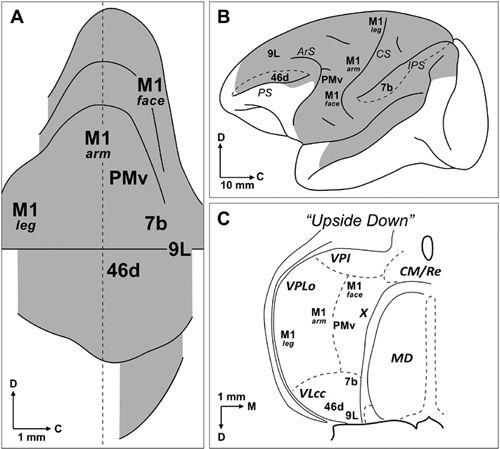
Figure 51. The cerebellothalamocortical circuit.
(A) A map of monkey dentate nucleus is unfolded with the dorsal surface upward and the caudal surface to the right side. (B) Selected cortical targets of cerebellothalamocortical circuits. Shading in this diagram indicates the cortical regions (lateral hemisphere only) that project to the cerebellum via the pons. (C) Origin of selected cortical projections from the ventrolateral thalamus. The cortical regions so indicated receive inputs from regions of the ventrolateral thalamus that lie within the termination zone of cerebellar efferents. The thalamus is turned upside down to indicate the match between its topography and that of the dentate nucleus. Abbreviations: 7b, 9L, 46d, Brodmann’s areas (see
Figure 2
); X, area X; ArS, arcuate sulcus; C, caudal; CM/Re, nucleus centrum medianum/nucleus reuniens; CS, central sulcus; D, dorsal; IPS, intraparietal sulcus; M, medial; M1, primary motor cortex: MD, nucleus medialis dorsalis; PMv, ventral premotor area; PS, principal sulcus; VLcc, caudal portion of the nucleus ventralis lateralis, pars caudalis; VPI, nucleus ventralis posterior inferior; VPLo, nucleus ventralis posterior lateralis, pars oralis. (From
Dum and Strick, 2003
.)
In summary, we explain the entire brain mechanism for learning motor actions by assuming a sequential combination of (1) an initial feedback-control phase during which a body/motor schema is being formed, (2) a conscious cerebral phase relying on the body/motor schema, and finally (3) an unconscious cerebellar phase wherein a forward and inverse internal model, which copied the body/motor schemata, are in operation.
In accordance with the preceding model, brain-imaging studies have demonstrated a co-activation of the parietal association cortex and the cerebellum during certain motor actions. For example, when fMRI brain images were compared for execution of a hand movement paced at 0.5 Hz by an auditory cue versus imagination (mental simulation) of the same movement at the same rate, it was shown that specific cortico-subcortical areas were more engaged in the mental simulation case, with the areas activated including the bilateral premotor, prefrontal, supplementary motor, and left posterior parietal areas and the caudate nuclei (
Gerardin et al., 2000
). In this study, the left parietal area was a likely candidate for representation of the body/motor schemata needed for the auditory-cued hand movements.
