The Cerebellum: Brain for an Implicit Self (34 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

When one is learning a novel voluntary movement, the required forward model must be reformulated by climbing fiber signals that represent errors and induce a persistent modification of its input-output relationship. In
Figure 43
, it is assumed that the inferior olive (IO) is driven by three types of input signal to the primary motor cortex that consist of 1) central instruction, 2) external feedback signals from a sensory system, and 3) internal feedback signals from the forward model. The presence of such triple pathways converging onto the IO is consistent with the three-phase climbing fiber discharges that occur during a monkey’s hand reaching movement (
Chapter 13
,
Section 3
).
Internal inverse models determine appropriate motor commands from the instruction they receive about the desired motor consequences. An interesting design was provided by the Kawato et al. (
1987
) unique two-degrees-of-freedom adaptive control for voluntary movement (
Figure 44
). It combined two controllers acting on a common controlled object that was composed of a spinal segmental motor system and its peripheral effector apparatus. The controller was a combination of the primary motor cortex, which mediated classic feedback control, and a microcomplex that provided feedforward control. The microcomplex acquired an inverse model of the controlled object by learning. Before this learning, feedback control by the primary motor cortex was dominant, but as learning proceeded in the microcomplex, feedforward control became predominant.
Figure 44. Inverse-model-based control system scheme for voluntary movements.
See the text for further information on this figure. The abbreviations are the same as those in
Figure 43
. d indicates descending tract neurons receiving inputs from both M1 and AIP. (Based on
Ito, 2001
,
2006
.)
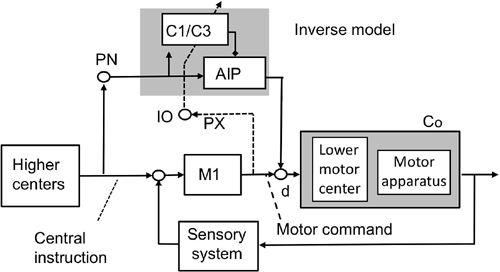
To act as an inverse model, a microcomplex must be connected with the primary motor cortex in a manner different to a forward model (compare
Figures 43
and
44
). Neurons in the supplementary motor cortex and the premotor cortex project to both the primary motor cortex (
Fang et al., 2005
) and the cerebellum via the pontine nucleus (
Wiesendanger et al., 1979
). Furthermore, the outputs of the primary motor cortex and the cerebellar interpositus nucleus converge on some descending tract neurons (d in
Figure 44
). For example, the rubrospinal tract neurons receive input from the anterior interpositus nucleus (
Toyama et al., 1970
) and also from the primary motor cortex (
Tsukahara and Kosaka, 1968
). Therefore, there seems to be an anatomical basis for postulating an inverse model for helping the primary motor cortex, but these connections need more detailed investigation.
For their two-degrees-of-freedom control system, Kawato et al. (
1987
) postulated that the most plausible possibility was that errors are derived from the outputs of the primary motor cortex, which perform feedback control to generate motor commands (feedback-error learning) (
Figure 44
). A computer simulation of this for a reaching movement reproduced Purkinje cell complex-spike discharges in two phases: an early response locked to movement onset (corresponding to the first phase; see above), which was always present, and a later response (corresponding to the third phase) that disappeared after learning, albeit the learning was stable and maintained (
Schweighofer et al., 1998
). Therefore, the temporal patterns of complex-spike discharges during movements should vary depending on which model (forward or inverse) is adopted in voluntary motor control (see
Section 15-4
).
In conformity with the feedback error-learning hypothesis, the primary motor cortex sends motor error signals to the IO via collaterals of the corticospinal tract and/or via the parvocellular red nucleus (
Chapter 6
, “
Pre- and Post-Cerebellar Cortex Neurons
”). Yttri et al. (
2006
) injected muscimol into the left parvocellular red nucleus of a monkey that had been trained to perform a right arm reaching task with and without 20 diopter Fresnel prisms that shifted the gaze 11.5° to the right. Parvocellular red nucleus inactivation did not affect the control (“no-prism”) gaze-reach calibration, but it impaired the learned calibration for right-shifting prisms. Moreover, the monkey was unable to adapt to stronger 30 diopter novel prisms that shifted the gaze 17.2° to the right. The parvocellular red nucleus appears to be necessary for the storage of error information from previous trials and the use of this error information to update motor programs in order to prevent an error in subsequent trials. Because this nucleus also receives inputs from the supplementary motor cortex, cingulate gyrus, frontal eye field, and posterior parietal area (
Ralston 1994
;
Burman et al., 2000a
,
b
), it may well mediate error learning in the cerebellum for both voluntary movements and motor actions (
Chapter 16
, “
Motor Actions and Tool Use
”).
The basic structures of forward- and inverse-model-based control systems are shown schematically in
Figures 8
,
43
, and
44
. To discuss how they differ in their operation, one must realize that instruction and sensory signals represent movement in spatial coordinates—that is, the description of motion of the body, or its various parts, in terms of position, velocity, acceleration, and direction (termed “kinematics”). In contrast, motor signals commanding movements are represented in bodily coordinates—that is, the description of motion that includes force as the cause of motion (termed “dynamics” or “kinetics”). In this volume, we prefer “dynamics” as it is used widely in internal-model-based control of a robot manipulator (
An et al., 1988
). In simple control systems, these two types of coordinates can be approximately identical; for example, for the VOR, the spatial coordinates determined by the semicircular canals parallel the bodily coordinates determined by the activation of extraocular muscles (
Ezure and Graaf, 1984a
;
Chapter 10
, “
Ocular Reflexes
”). In complex control systems, however, such as those required for multijoint arm/hand/finger movements, the bodily coordinates are complex and clearly distinct from spatial coordinates. Therefore, sensory and motor signals need to be represented in their spatial and bodily coordinates, respectively, but not in their admixture. Here, we address the question of what rules determine the sensory/motor nature of mossy and climbing fiber information, and the nuclear output of a microcomplex.
It follows that a forward model receives motor signals from a controller and generates sensory signals that provide information on the kinematics of the produced movement. In contrast, an inverse model receives instruction signals in spatial coordinates and generates motor commands like a controller. Therefore, the forward/inverse nature of a microcomplex can be distinguished by determining whether the output of this microcomplex represents kinematics or dynamics. It has been shown that Purkinje cells in lobules HIV–HVI of the intermediate and lateral parts of the cerebellar hemisphere encode the position of an arm and the direction and speed of its movements, thereby favoring the forward model (
Fu et al., 1997
:
Roitman et al., 2005
). Another similar example is grip force-load force coordination in holding a load stationary in space (
Chapter 13
). An fMRI study revealed the brain activity related to this coordination and demonstrated that the cerebellum was the most likely site for forward models to be stored (
Kawato et al., 2003
). After reviewing the data in this field, Ebner and Pasalar (
2008
) concluded that the simple-spike discharges of Purkinje cells do not have dynamics-related signals in keeping with an inverse dynamics model. However, Yamamoto et al. (
2007
) detected muscle dynamics representation in a group of Purkinje cells in lobules HIV–HVI of the intermediate parts of the cerebellar hemisphere. These Purkinje cells formed a group separate from other Purkinje cells that represented movement kinematics. These findings suggested that both forward and inverse models are utilized in the control of arm movements. However, it is desirable to test more cases of voluntary
motor control to clarify how these two types of internal model are differentially utilized for voluntary motor control. It is also important to test the kinematic/dynamic nature of the activity of cerebellar/vestibular nuclear neurons, but not Purkinje cells, because the former provide the real output signals of internal models (as discussed in
Chapter 12
, “
Adaptive Control System Models
”).
The forward and inverse models can also be distinguished by the difference in the anatomical design of control systems incorporating a microcomplex. If one wants to provide a forward model, it is essential to have a microcomplex within a cerebrocerebellar communication loop. Microcomplexes involving C
1
and C
3
zones are indeed linked in a loop with the primary motor cortex and hence are able to provide forward models (
Figure 43
). In contrast, microcomplexes located in the cerebellar hemisphere and involved in voluntary eye movements have no connections to the frontal eye field (Kelly and Strick, 2002). Hence, the cerebellar hemisphere cannot provide forward models to controllers in the frontal eye field. Possibly, the cerebellar hemisphere provides inverse models to the frontal eye field in the manner similar to that shown in
Figure 44
for M1. This possibility is in accordance with a functional feature of eye movement, which, in particular for the saccades, is so fast that it cannot be controlled by internal feedback through a forward model.
These considerations suggest an interesting dichotomy that voluntary limb movements are controlled with forward models, whereas inverse models control voluntary eye movements. This suggestion is worth testing by designing experiments that involve further recording from the cerebellum.
The mixed sensory/motor nature of climbing fiber signals has been observed in the VOR and OFR (
Chapter 10
). In voluntary movement, climbing fibers convey either sensory or motor signals depending on the nature of the internal model involved: climbing fibers convey sensory signals to a forward model (
Figure 43
) and motor signals to an inverse model (
Figure 44
). In the decorrelation control algorithm proposed by Dean et al. (2002) and Porrill et al. (2004) a combination of motor signals in mossy fibers and sensory signals in climbing fibers produced efficient learning. If a positive correlation induces LTD and a negative correlation produces LTP, a cerebellar microcomplex can learn effectively even without monitoring motor errors. This situation applies to the forward model (
Figure 43
), but not to the inverse model (
Figure 44
). For the latter, Gomi and Kawato (1993) applied a feedback error-learning rule (see previous discussion). These two rules
are incompatible with each other because the former involves the sensory nature of climbing fiber activity, whereas the latter emphasizes its motor nature.
What signals do climbing fibers convey in an actual voluntary movement? In a well-known study (
Kitazawa et al., 1998
), a monkey performed short-lasting reaching by moving the hand and arm (without seeing them) to touch a visual target that appeared at a random location on a screen. Complex Purkinje cell spikes occurred in three successive response phases. The spikes in the third phase ceased at the end of the reaching movements, suggesting that they represented visually perceived deviations between the instructed target and the reached finger position (consequence errors). The second phase appeared near the moment of reaching but too early to reflect the consequence of reaching. These spikes presumably reflected deviations between the instructed target and the target position predicted by the forward model (i.e., internal errors). This second phase is in keeping with forward model control equipped with an internal feedback (
Figure 43
), but it has no basis in terms of inverse model control without an internal feedback (
Figure 44
).
The first phase of the preceding discharges occurred when the monkey initiated the reaching movement. At that moment, the cerebral cortex was being driven by instruction about the need for the reaching movement toward the visual target. The difference between the position of the ready-to-go hand and the instructed target position can be considered to be an “initial set error.” Alternatively, this error might represent the discrepancy between the target position actually viewed and that which the monkey anticipated from preceding experiences. This would be the case if the monkey had learned previously to reach a target that appeared repeatedly at the same position. In the experiment under consideration, however, the target’s position was randomized from trial to trial to prevent this type of learning. Therefore, the earliest complex-spike discharges in the Kitazawa et al. (
1998
) study were more likely to have reflected the first alternative: that is, the difference between the positions of the hand and target immediately prior to the reaching movement.
