The Cerebellum: Brain for an Implicit Self (9 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

In the 1970s, functional roles of the cerebellum were under intense discussion. Even though the role of the cerebellum in the control of body equilibrium, the finger-nose test, and arm retraction had been proposed in classic studies, mechanisms underlying these roles seemed too complex to analyze experimentally. Jun Fukuda,
Stephen Highstein, and I searched for a simple system to analyze. We found that the vestibuloocular reflex (VOR) was an appropriate experimental model. In response to a head movement sensed by the vestibular organ, the VOR produces an eye movement to maintain stable retinal images during head movement. We first showed that the VOR was inhibited directly by Purkinje cells located in the flocculus, that is, in the phylogenetically oldest part of the cerebellum (
Fukuda et al., 1972
;
Kawaguchi, 1985
). This finding was the beginning of our flocculus hypothesis for VOR adaptation (
Ito, 1972
,
1974
,
1982
) and continuing debate about its mechanism, as will be introduced in
Chapters 10
and
12
.
Importantly, VOR is a feedforward control system that has no feedback from output to input; that is, there is no way to inform the vestibular system directly about eye movement (
Ito, 1974
). In engineering systems, feedforward control alone is undesirable because without feedback, the control cannot be precise. However, in biological systems, feedback may not always be available. In such cases, another CNS pathway (or pathways) is needed to replace the traditional feedback loop. We reasoned that this could be the flocculus. Our assertion was supported by the observation that the vestibulospinal reflex held head position constant by using direct feedback from the neck’s position to the vestibular organ but using no cerebellar inhibition. For the VOR to obtain precise compensatory eye movements without feedback, there had to be a visual pathway to the flocculus that informed about errors in the operation of the reflex (
Ito, 1970
). To test this prediction, the late Kyoji Maekawa (1929–1990) and John Simpson (
Maekawa and Simpson, 1973
) indeed discovered in my laboratory a powerful climbing fiber projection from the retina to the flocculus. Input to the flocculus from the vestibular organ via mossy fibers had already been shown in the cat (
Brodal, 1972
). In summary, our VOR model incorporated a set of three elements of the Marr-Albus model: mossy fiber-parallel fiber input, climbing fiber input, and Purkinje cell output. Also, the VOR was testable in a behaving animal!
The XXVth Congress of the IUPS was held in Munich in 1971. It was an unforgettable experience for me. I reported about the direct inhibition of VOR relay neurons by flocculus Purkinje cells and proposed that the flocculus plays a key role in the feedforward control of the VOR. To my great surprise in this same session, Geoffrey Melvill Jones reported that when a human subject wore Dove-prism goggles, which reversed the right-left relationship in the visual field for one month, the result was a clear-cut depression and final reversal of the VOR (
Gonshor and Melvill Jones, 1974
). David Robinson, a world-renowned oculomotor physiologist/bioengineer, was leading discussions in this session. After the Congress, he attached Dove-prism goggles to a kitten and showed that the VOR was substantially depressed (
Robinson, 1976
). Moreover, he showed that the depression did not occur when the flocculus had been lesioned bilaterally. I learned the “double rotation” technique (use of vestibular and visual stimuli in various combinations) from an otologist, who was working on vestibular functions in race-car drivers, and applied it to rabbits. We oscillated them sinusoidally on a horizontal turntable and also moved a surrounding screen horizontally (
Ito et al., 1974
). When the screen was rotated in the direction opposite to the turntable rotation, the VOR was gradually enhanced (i.e., to catch up with the increased relative movement of the screen). Likewise, while the screen was rotated in the same direction as the turntable, the VOR was gradually depressed. These adaptive changes in the VOR were abolished when the flocculus was ablated bilaterally (
Batini et al., 1979
) or when climbing fibers were lesioned bilaterally (
Ito and Miyashita, 1975
). We then proceeded to record from flocculus Purkinje cells during VOR adaptation (
Ghelarducci et al., 1975
;
Dufosse et al., 1978
). Since that time, numerous such studies have been carried out in many laboratories, but nonetheless, VOR adaptation remains a valuable system for investigating mechanisms of cerebellar motor control and such work still generates new issues in cerebellar research (
Chapter 10
).
A frequently discussed question in the late 1960s and early 1970s was what signals climbing fibers conveyed as a set of unique afferents to the cerebellum. Marr (
1969
) assumed that they provided instruction signals from the cerebral cortex, whereas Albus (
1971
) thought that climbing fiber input implied errors in the simple perceptron-like operation of a cerebellar network. Miller and Oscarsson (
1970
) proposed that the inferior olive acted as a comparator between command signals from higher centers and the activity these signals evoked at lower levels. I proposed that climbing fibers monitored “control errors” for the VOR (
Ito, 1970
). Amat (
1983
) observed in the frog cerebellum that climbing fibers responded to a shift in the position of a forelimb and suggested that these responses represented a deviation of the forelimbs from a predetermined position. Since then, the signal contents of climbing fiber discharges have been investigated extensively. It seems to be a general principle that climbing fiber signals encode errors of some sort; not always an error occurring as a consequence of a movement, but also an error generated intrinsically within a neuronal circuit (
Chapter 13
, “
Voluntary Motor Control
”). When climbing fibers convey error signals, LTD would be induced in those parallel fiber-Purkinje cell synapses that are involved in erroneous performance. Learning would then occur to reduce such incorrect behavior (i.e., “error learning”). This notion has been expanded to motor learning in general, and it is sometimes called Marr-Albus-Ito hypothesis.
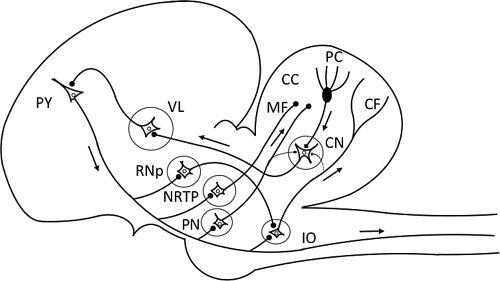
Also about 40 years ago, I speculated about the function of a then well-known anatomical structure of the cerebellum, the cerebrocerebellar loop that links the primary motor cortex and the intermediate part of the cerebellar hemisphere (
Figure 18
). Initially, I failed to relate this loop to the circuit structure I had proposed for VOR adaptation. Therefore, I then introduced the idea that the cerebellum provided an internal model that helped the cortical controllers. The idea was as follows. In performing unskilled voluntary movements, the initial instruction arising from an association area of the cerebral cortex would be transferred to the primary motor cortex and then through the pyramidal tract down to the spinal motor centers. The final outcome would be checked through sensory pathways by the association cortex, there being a large negative feedback loop formed through the external world. In this case, the cerebral cortex had to be continuously aware of what was being performed and had to be available for adjusting the performance from time to time. As experience was gained in the performance of these movements, they would become refined to the level of being skilled voluntary movements. As the learning process progressed, it was suggested that the large loop through the external world would be effectively replaced by an internal loop passing through the cerebellum, such that it would serve as a model simulating the combination of the spinal control system, the external world, and the sensory pathways. In this gestalt, the original negative feedback system would be converted by learning into a feedforward system that needed no straightforward negative feedback from the output to the input. I submitted an invited manuscript on this idea of an internal model to the 4th Symposium of the Fulton Society on the Cerebellum, which was held in New York City in 1969. Unfortunately, an illness prevented me from attending the meeting, but the manuscript was nonetheless circulated among the participants and eventually published in a journal that collected publications concerning that meeting (
Ito, 1970
). I also presented the idea in my 1984 monograph,
The Cerebellum and Neural Control
.
Figure 18. The cerebrocerebellar loops.
This figure schematizes the loop connections between the cerebral cortex and the cerebellum. Abbreviations: CC. cerebellar cortex; CF, climbing fiber; CN, cerebellar nucleus; IO, inferior olive; MF, mossy fiber; NRTP, nucleus reticularis tegmenti pontis; PC, Purkinje cell; PN, pontine nucleus; Py, pyramidal cell in the cerebral cortex; RNp. parvocellulr red nucleus; VL, ventrolatertal thalamic nucleus.
In the 1980s, movements of multijoint robotic fingers, arms, and hands became a challenging control task because such movements have a large number of degrees of freedom (
Chapter 13
). Hollerbach (
1982
) and An et al. (
1988
) introduced a clever way of controlling a robot’s arm using feedforward control via an inverse model of the arm. In 1987, Mitsuo Kawato and his colleagues proposed an ingenious two-degrees-of-freedom control, in which the feedback control by the primary motor cortex was combined with feedforward control by the cerebellum (
Figure 8B
). If the cerebellum represented the output-input relationship of the controlled object, this inverse model could play the role of a feedforward controller. For this system, Kawato et al. (
1987
) incorporated an ingenious way of learning, that is, “feedback error learning” that derived errors from the primary motor cortex performing its feedback control. The Kawato model seemed to be an effective way to explain the learning process in voluntary motor control. Initially, the primary motor cortex would exert feedback control to perform accurate movements. Meanwhile, the cerebellar inverse model would gradually be modified by error signals to provide precise feedforward control. Then the feedback control by the primary motor cortex would be replaced by feedforward control from the cerebellum unless the latter happened to be inaccurate. It could be reasoned that the initial feedback control was performed consciously, whereas the later feedforward control by the cerebellum is performed unconsciously, this idea being in good general agreement with our daily experiences. Moreover, a combination of forward and inverse models was applied successfully to the creation of a robot that was able to learn movement skills (
Wolpert and Kawato, 1989
). A major advantage of Kawato’s control system model was its computational expression, such that it could be installed in a robot that was capable of learning complex movements. The biological validity of the forward and inverse models is now being tested in an ever-increasing number of experimental studies on Purkinje cell discharges during various movement paradigms (
Chapter 15
, “
Internal Models for Voluntary Motor Control
”).
A major new development in cerebellar research toward cognitive functions began in the early 1990s. A role for the cerebellum was postulated for cognitive functions, such as language acquisition, on the basis of the considerable expansion of the most lateral part of the cerebellar hemispheres in humans (Leiner et al., 1993). Clinical observations had also shown by then that lesions in the cerebellum often accompanied mental and affective disorders with characteristic symptoms, which Schmahmann (
1991
) called mental dysmetria. In spite of these important developments, the general line of thought at that time was rather negative (e.g.,
Leiner, 2010
).
At an earlier time, the late Robert Dow (1908–1995) asked me to write an article on the cerebellum for a special issue of Trends in Neuroscience in 1993. I reflected on how cerebellar neuronal circuits might process information not only for body movements in the physical domain but also for conceptual functions in the cognitive domain. I was inspired with the idea that in the control systems gestalt, the control of a body part is analogous to manipulation of a mental model, like those proposed by Craik (
1943
) and Johnson-Laird (
1983
), and even by Piaget’s (
1951
) schema (
Chapter 1
).
I presented this view in the Pontifical Academy of Sciences Symposium organized by John Eccles and the late Otto Detlev Creutzfeldt (1927-1992) at the Vatican in 1988. The overall reaction to my talk (Ito, 1990a) was that the hypothesis was interesting, but had no supportive data. Decety encouraged me, however, by showing a PET image of the cerebellum of a tennis player playing the game in his mind without making any movement (i.e., an image training paradigm) (Decety et al., 1990). In the following years, I developed the view that the cerebellum controls movements and thoughts with the same overall neuronal circuit mechanisms (Ito,
1993a
,
1997b
). I further speculated that a presumed mental model is initially formed in the temporoparietal cortex, and as learning proceeds, it is copied in cerebellar internal models (
Ito, 2005
).
