The Cerebellum: Brain for an Implicit Self (7 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

As a harbinger of the coming breakthrough in the 1960s, some important findings were reported in the 1950s. Ragner Granit (1900–1991) and Charles Phillips (1916–1994) used microelectrode recording to demonstrate the antidromic spikes of Purkinje cells and the so-called D potentials. The latter corresponded to intracellularly recorded climbing fiber responses (
Granit and Phillips, 1956
). Janos Szentágothai (1912–1994) and coworkers identified the origin of climbing fibers in the IO. They surmised from the pattern of connections among granule cells, Purkinje cells, and basket cells that basket cells were inhibitory neurons (
Szentágothai and Palkovits, 1959
;
Szentágothai, 1963
).
Morphological studies revealed evolution-related structural-functional maps of the cerebellum, and lesion studies established the specific involvement of the cerebellum in the learning of precise movements. These studies laid a firm foundation for the modern approach to the cerebellum, which focuses on neuronal circuits that are discussed in later chapters.
In the 1960s, the cerebellum was considered to be an elaborate neuronal machine composed of intricate neuronal circuits with geometrical refinement. It was thought to process information that was critical for the acquisition of motor skills. During the five subsequent decades, research on the cerebellum has been devoted largely to addressing questions about how its neuronal circuits are constructed and function, and what specific roles they play. The principles of modern systems control, particularly adaptive and model-based control, have been introduced. Furthermore, the role of the cerebellum in the manifestation of intelligence is now under consideration. This progress is summarized in the following sections, including some of my personal experiences and impressions throughout this 50-year period.
In the 1960s, Professor John Eccles, who had discovered inhibitory synapses in the spinal cord (
Brock et al., 1952
), turned to the study of the cerebellum with his talented colleagues in Canberra, Australia. It was a remarkable time when electrophysiology with glass microelectrodes enabled intracellular recording in individual neurons. Using this technology, Eccles distinguished two types of neurons, excitatory and inhibitory, in the cat spinal cord (
Figure 3A
,
B
). Excitatory neurons were shown to supply solely excitatory synapses and induce excitatory postsynaptic potentials (EPSPs) or currents (EPSCs) in their target neurons. In contrast, inhibitory neurons were shown to supply inhibitory synapses that induced inhibitory postsynaptic potentials (IPSPs) or currents (IPSCs) in their targets. Using the same technology and taking advantage of the geometrical arrangement in cerebellar circuits, Eccles and his associates quickly identified basket cells, stellate cells, and Golgi cells as inhibitory neurons, and granule cells, mossy fibers, and climbing fibers as excitatory elements (
Chapters 4
and
5
).
Here, let me recall my first experience with the cerebellum. In 1962, I returned to Tokyo from Eccles’ laboratory where I had studied spinal motoneurons for three years. On my return I worked with several colleagues on two types of giant neurons in the brainstem. These were Otto Deiters’ (1834–1863) giant neurons (
Deiters, 1865
; see
Mazzarello, 1999
) and magnocellular red nucleus neurons. I was familiar with these neurons from the earlier anatomy lectures of Professor Teizo Ogawa (1901–1984), which I had heard while a medical student. We equipped one laboratory exclusively with hand-made electronic instruments. It was shared by two subgroups: the late Nakakira Tsukahara (1933–1985) and Kesiuke Toyama for the red nucleus and the late Mitsuo Yoshida (1933–1998) and me for Deiters neurons. In November 1963, we were successful in recording intracellularly in a Deiters neuron of an anesthetized cat. When we applied an electric shock to needle electrodes inserted into the cerebellum, it caused a large swing of green spots on the screen of a cathode ray oscilloscope. This was an IPSP induced via long axons of Purkinje cells (
Figure 13
) (
Ito and Yoshida, 1964
). We then recorded from cerebellar nuclear neurons and confirmed the consistent occurrence of inhibition, thereby enabling our conclusion that Purkinje cells were uniformly inhibitory neurons (
Ito et al., 1964
). Moreover, when Kunihiko Obata joined us a short time later, we found that iontophoretic application of gamma-amino-butyric acid (GABA) to Deiters neurons induced a membrane hyperpolarization like IPSPs (
Obata et al., 1967
). This evidence showed that Purkinje cells were GABA-releasing inhibitory neurons. At that time, there were the beliefs that (1) large neurons with long axons were excitatory, whereas small neurons with short axons were inhibitory; and (2) excitatory neurons were major “players” in the brain, whereas inhibitory neurons acted as “local commutators.” Indeed, the inhibitory neurons identified by Eccles and his colleagues in the spinal cord, hippocampus, and cerebellar cortex were all short-axoned, relatively small neurons. Our Purkinje cell finding was also at variance with the then-conventional thought that the cerebellum was involved in both excitatory and inhibitory functions because its stimulation induced either contraction or relaxation of limb muscles, as dependent on the stimulation conditions. We showed, however, that target neurons for Purkinje cell inhibition receive excitation via axon collaterals of mossy fiber and climbing fiber afferents (
Figure 13
) (
Ito et al., 1969
). Morphological details of such axon collaterals were revealed later (
Shinoda et al., 1992
;
Sugihara et al., 1996
). We found also that stimulation of the cerebellum often facilitated Deiters neurons via inhibition of Purkinje cell inhibition—that is, disinhibition (
Ito et al., 1968
). When these controversies subsided, Eccles generously offered me the opportunity to co-write with Szentágothai and him the 1967 monograph,
The Cerebellum as a Neuronal Machine
.
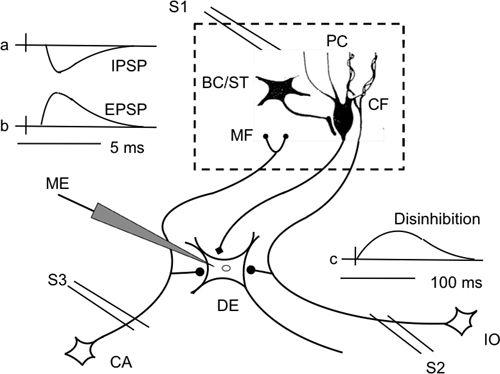
Figure 13. Schematic neuronal circuit showing how electrical stimulation of the cerebellar cortex induces three major effects in Deiters neurons.
When recorded intracellularly in a Deiters giant neuron (DE), electrical stimulation of Purkinje cells induces inhibitory postsynaptic potentials (IPSPs)(a). However, excitatory postsynaptic potentials (EPSPs) are also induced by activation of mossy fibers and climbing fibers via their axon collaterals (b). If basket cells are stimulated, they inhibit Purkinje cells so that Deiters neurons are disinhibited and generate slow disibhibitory depolarization (c). Part of the neuronal circuit located in the cerebellar cortex is enboxed by broken lines. Abbreviations: CA, cells of origin of mossy fibers (MF); IO, inferior olive that issues climbing fibers (CF); ME, microelectrode; PC, Purkinje cells. Note that both CF and MF project collaterals to the Deiters cells. (Based on the data of
Ito and Yoshida, 1966
,
Ito et al., 1968
,
1969
.)
We wrote this book at a time when computers were beginning to be used widely in neuroscience and artificial intelligence seemed of particular promise. After Wiener popularized cybernetics in his 1948 book, modern control theories had appeared to be a promising approach for advancing understanding of the mechanisms of the CNS. For example, Arbib (
1971
) applied cybernetic concepts to brain theories. Our 1967 monograph emphasized wiring diagrams of the cerebellum, and we encouraged computational scientists to collaborate with biological researchers to determine their significance. At the end of the book, we stated confidently that the enlightened discourse between such theorists on the one hand and neurobiologists on the other will lead to the development of revolutionary hypotheses of the way in which the cerebellum functions as a neuronal
machine and predicted that these hypotheses will lead to revolutionary developments of experimental investigation (
Eccles et al., 1967
).
Several international symposia were held with a focus on this theme. The most impressive one for me was held in 1967 at Salishan Lodge near Gleneden Beach on the Oregon coastline, USA, as organized by Francis Schmitt (1903–1995) and Eccles. Donald MacKay (1922–1987) led discussions among theorists, computer experts, and bioengineers. There was no immediate outcome from this and other such meetings, however. Despite the impressive beauty of its wiring diagrams (Color Plate V), the “neuronal machine” concept of the cerebellum remained vaguely defined as “a relatively simple machine devoted to some essential information processing.” I was frustrated enough at the Salishan meeting to ask what else experimentalists would need to uncover before we would be able to understand the meaning of these wiring diagrams. Someone equally frustrated replied that the available diagrams were too simple to construct even a primitive radio, so more information was urgently needed before any meaningful model could be conceived. However, an important clue had already been with us for a long time—that is, the presence of climbing fibers in the cerebellum, as described by Cajal (
1911
) (
Figure 14
). The contrasting connectivity of each Purkinje cell with only one
climbing fiber and numerous parallel fibers had been interpreted only as unique cases of convergence and divergence. Characteristic electrical events induced in a Purkinje cell by impulses of parallel fibers and climbing fibers (as exemplified in
Figure 15
) had been revealed by Eccles et al (
1966a
,
b
) and Thach (
1967
), but no one thought of its implication for synaptic plasticity except Brindley (
1964
) who pointed out this possibility.
Figure 14. Convergence of climbing and parallel fibers onto Purkinje cells.
A part of Figure 104 of Cajal (
1911
) is shown with the right, left axis reversed to match Color Plate V. A, mossy fiber; B, Purkinje cell axon; a, granule cell; b, parallel fiber; c, Purkinje cell; d, climbing fiber. Arrows indicate the supposed directions of signal flow.
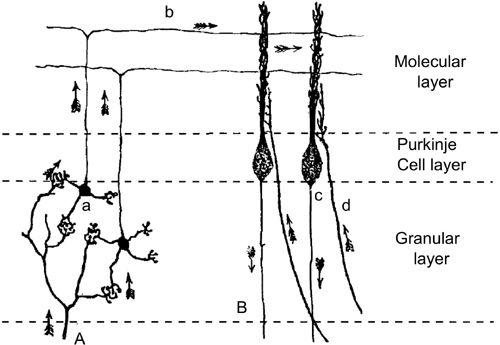
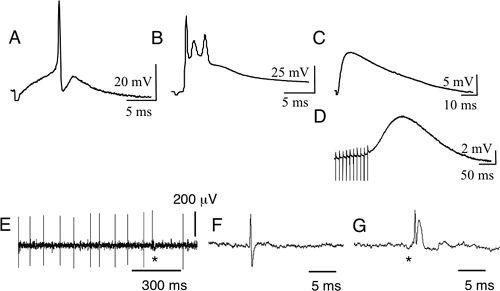
Figure 15. Bioelectric potentials of Purkinje cells.
Both intracellular recordings in slices (A–D) and extracellular recordings in vivo (E–G) are shown. (A) Simple spikes induced by stimulation of parallel fibers (PFs) on the pial surface of a cerebellar folium. (B) Complex spikes evoked by stimulation of climbing fibers in the white matter. The complex potentials so evoked are composed of an EPSP and Na
+
and Ca
2+
spikes. (C) An AMPA-EPSP evoked by stimulation of parallel fibers. (D) mGluR-EPSPs evoked by repetitive stimulation of parallel fibers in the presence of an AMPA antagonist. (E) Spontaneous discharge from a Purkinje cell. (F) A simple spike in an expanded time scale. (G) A complex spike similarly shown. In A–D, five consecutive sweeps repeated at 0.2 Hz were averaged. (From unpublished data of Le and Ito.)