The Coming Plague (131 page)
Authors: Laurie Garrett

96
J. Davies, “Inactivation of Antibiotics and the Dissemination of Resistance Genes,”
Science
264 (1994): 375â82; and B. G. Spratt, “Resistance to Antibiotics Mediated by Target Alternatives,”
Science
264 (1994): 388â93.
Science
264 (1994): 375â82; and B. G. Spratt, “Resistance to Antibiotics Mediated by Target Alternatives,”
Science
264 (1994): 388â93.
97
M. Raymond, P. Gros, M. Whiteway, and D. Y. Thomas, “Functional Complementation of Yeast ste6 by a Mammalian Multidrug Resistance
mdr
Gene,”
Science
256 (1992): 232â34.
mdr
Gene,”
Science
256 (1992): 232â34.
98
C. F. Amabile-Cuevas and M. E. Chicurel, “Horizontal Gene Transfer,”
Scientific American
81 (1993): 332â41.
Scientific American
81 (1993): 332â41.
99
M. Blot, J. Meyer, and W. Arber, “Bleomycin-Resistance Gene Derived from the Transposon
TnS
Confers Selective Advantage to
Escherichia coli
K-12,”
Proceedings of the National Academy of Sciences
88 (1991): 9112â16.
TnS
Confers Selective Advantage to
Escherichia coli
K-12,”
Proceedings of the National Academy of Sciences
88 (1991): 9112â16.
100
For an excellent overview of the various genes inside normal bacterial chromosomes that control the absorption and use of mobile DNAs, see D. J. Galas and M. Chandler, “Bacterial Insertion Sequences,” Chapter 4 in Berg and Howe (1989), op. cit.
101
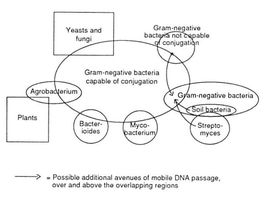
Amabile-Cuevas and Chicurel [(1992), op. cit.] pictured the movement of transposons and plasmids between various families of organisms as a highly fluid and ongoing process. On the basis of organism interactions and the relatedness of various known plasmids and transposons, they came up with the following representation of likely gene swapping.

102
J. D. Boeke, “Transposable Elements in
Saccharomyces cerevisiae
,” Chapter 13 in Berg and Howe (1989), op. cit.; and H. Varmus and P. Brown, “Retroviruses,” Chapter 3 in Berg and Howe (1989), op. cit.
Saccharomyces cerevisiae
,” Chapter 13 in Berg and Howe (1989), op. cit.; and H. Varmus and P. Brown, “Retroviruses,” Chapter 3 in Berg and Howe (1989), op. cit.
103
R. Saral, W. H. Burns, O. L. Laskin, et al., “Acyclovir Prophylaxis of Herpes-Simplex-Virus Infections: A Randomized, Double-Blind, Controlled Trial in Bone-Marrow-Transplant Recipients,”
New England Journal of Medicine
305 (1981): 63â67.
New England Journal of Medicine
305 (1981): 63â67.
104
G. J. Mertz, C. W. Critchlow, J. Benedetti, et al., “Double-Blind Placebo-Controlled Trial of Oral Acyclovir in First-Episode Genital Herpes Simplex Virus Infection,”
Journal of the American Medical Association
252 (1984): 1147â51; and S. E. Straus, H. E. Takiff, M. Seidlin, et al., “Suppression of Frequently Recurring Genital Herpes,”
New England Journal of Medicine
310 (1984): 1545â50.
Journal of the American Medical Association
252 (1984): 1147â51; and S. E. Straus, H. E. Takiff, M. Seidlin, et al., “Suppression of Frequently Recurring Genital Herpes,”
New England Journal of Medicine
310 (1984): 1545â50.
105
K. E. VanLandingham, B. Marsteller, G. W. Ross, and F. G. Hayden, “Relapse of Herpes Simplex Encephalitis After Conventional Acyclovir Therapy,”
Journal of the American Medical Association
259 (1988): 1051â53; A. L. Rothman, S. H. Cheeseman, S. N. Lehrman, et al., “Herpes Simplex Encephalitis in a Patient with Lymphoma: Relapse Following Acyclovir Therapy,”
Journal of the American Medical Association
259 (1988): 1056â57; and R. J. Whitley, “The Frustrations of Treating Herpes Simplex Virus Infections of the Central Nervous System,”
Journal of the American Medical Association
259 (1988): 1067.
Journal of the American Medical Association
259 (1988): 1051â53; A. L. Rothman, S. H. Cheeseman, S. N. Lehrman, et al., “Herpes Simplex Encephalitis in a Patient with Lymphoma: Relapse Following Acyclovir Therapy,”
Journal of the American Medical Association
259 (1988): 1056â57; and R. J. Whitley, “The Frustrations of Treating Herpes Simplex Virus Infections of the Central Nervous System,”
Journal of the American Medical Association
259 (1988): 1067.
106
W. I. Whittington and W. J. Cates, Jr., “Acyclovir Therapy for Genital Herpes: Enthusiasm and Caution in Equal Doses,”
Journal of the American Medical Association
251 (1984): 2116â17.
Journal of the American Medical Association
251 (1984): 2116â17.
107
Though acyclovir was first used to treat herpes simplex-2, which caused genital herpes, it soon proved effective in controlling the entire family of herpes viruses, including varicella (chicken pox and shingles), herpes zoster, and herpes simplex-1. All of these viruses had the ability to hide latently inside human nerve cells for years, even decades, only surfacing when immunological conditions in the host favored their survival. For example, the same virus that caused childhood chicken pox would hide for five or six decades, resurfacing to produce often excruciating shingles.
108
L. Seale, C. J. Jones, S. Kathpalia, et al., “Prevention of Herpesvirus Infections in Renal
Allograft Recipients by Low-Dose Oral Acyclovir,”
Journal of the American Medical Association
254 (1985): 3435â38.
Allograft Recipients by Low-Dose Oral Acyclovir,”
Journal of the American Medical Association
254 (1985): 3435â38.
109
D. Parris and J. E. Harrington, “Herpes Simplex Virus Variants Resistant to High Concentration of Acyclovir Exist in Clinical Isolates,”
Antimicrobial Agents and Chemotherapy
22 (1982): 71â77.
Antimicrobial Agents and Chemotherapy
22 (1982): 71â77.
110
E. Katz, O. Rosenblat, and S. Pisanty, “Isolation and Characterization of Herpes Simplex Virus Resistant to Nucleoside Analogs,”
Oral Surgery, Oral Medicine and Oral Pathology
72 (1991): 296â99.
Oral Surgery, Oral Medicine and Oral Pathology
72 (1991): 296â99.
111
H. J. Field and S. E. Goldthorpe, “The Pathogenicity of Drug-Resistant Variants of Herpes Simplex Virus,” Fourth Forum in Virology, 1992, 120â24.
112
K. S. Erlich, J. Mills, P. Chatis, et al., “Acyclovir-Resistant Herpes Simplex Virus Infections in Patients with the Acquired Immunodeficiency Syndrome,”
New England Journal of Medicine
320 (1989): 293â96; and R. J. Whitley and J. W. Gnann, Jr., “Acyclovir: A Decade Later,”
New England Journal of Medicine
327 (1992): 782â89.
New England Journal of Medicine
320 (1989): 293â96; and R. J. Whitley and J. W. Gnann, Jr., “Acyclovir: A Decade Later,”
New England Journal of Medicine
327 (1992): 782â89.
113
Quite unfortunately, the sexual partner refused to cooperate with the study, so Straus was unable to absolutely confirm this hypothesis by performing PCR analysis of his herpes strain. It is a sorry fact that individuals commonly decline to participate in such studies, which could prove of immense good for the community as a whole. Such lack of participation is evident in all types of people. The failure of cooperation in this caseâin a gay manâwas actually fairly unusual, as the American gay community had proven remarkably open to scientists and their investigations since the onset of the AIDS epidemic.
114
The study is described in R. G. Kost, E. L. Hill, M. Tigges, and S. Straus, “Brief Report: Recurrent Acyclovir-Resistant Genital Herpes in an Immunocompetent Patient,”
New England Journal of Medicine
329 (1993): 1777â81.
New England Journal of Medicine
329 (1993): 1777â81.
115
S. Safrin, C. Crumpacker, P. Chatis, et al., “A Controlled Trial Comparing Foscarnet with Vidarabine for Acyclovir-Resistant Mucocutaneous Herpes Simplex in the Acquired Immunodeficiency Syndrome,”
New England Journal of Medicine
325 (1991): 551â55; and S. Safrin, “Management of Patients Following Successful Healing of Acyclovir-Resistant Herpes Simplex Infection,” Fourth Forum on Virology, 1992, 125â26.
New England Journal of Medicine
325 (1991): 551â55; and S. Safrin, “Management of Patients Following Successful Healing of Acyclovir-Resistant Herpes Simplex Infection,” Fourth Forum on Virology, 1992, 125â26.
116
J. M. Pepin, F. Simon, M. C. Dazza, and F. Brun-Vezinet, “The Clinical Significance of
in vitro
Cytomegalovirus Susceptibility to Antiviral Drugs,” Fourth Forum on Virology, 1992, 126â27; and C. Leport, S. Puget, J. M. Pepin, et al., “Cytomegalovirus Resistant to Foscarnet: Clinicovirologic Correlation in a Patient with Human Immunodeficiency Virus,”
Journal of Infectious Diseases
168 (1993): 1329â30.
in vitro
Cytomegalovirus Susceptibility to Antiviral Drugs,” Fourth Forum on Virology, 1992, 126â27; and C. Leport, S. Puget, J. M. Pepin, et al., “Cytomegalovirus Resistant to Foscarnet: Clinicovirologic Correlation in a Patient with Human Immunodeficiency Virus,”
Journal of Infectious Diseases
168 (1993): 1329â30.
117
N. S. Lurain, K. D. Thompson, E. W. Holmes, and G. S. Read, “Point Mutations in the DNA Polymerase Gene of Human Cytomegalovirus That Result in Resistance to Antiviral Agents,”
Journal of Virology
66 (1992): 7146â52.
Journal of Virology
66 (1992): 7146â52.
118
S. Safrin, S. Kemmerly, B. Plotkin. et al., “Foscarnet-Resistant Herpes Simplex Virus Infection in Patients with AIDS,”
Journal of Infectious Diseases
169 (1994): 193â96.
Journal of Infectious Diseases
169 (1994): 193â96.
119
M. C. Y. Heng, S. Y. Heng, and S. G. Allen, “Co-infection and Synergy of Human Immunodeficiency Virus-1 and Herpes Simplex-1,”
Lancet
343 (1994): 255â58.
Lancet
343 (1994): 255â58.
120
The literature on AZT resistance is vast and occasionally contradictory on questions of timing of emergence. Key studies include: A. Erice, D. L. Mayers, D. G. Strike, et al., “Brief Report: Primary Infection with Zidovudine-Resistant Human Immunodeficiency Virus Type 1,”
New England Journal of Medicine
328 (1993): 1163â65; M. S. Hirsch and R. T. D'Aquila, “Therapy for Human Immunodeficiency Virus Infection,”
New England Journal of Medicine
328 (1993): 1686â95; V. A. Johnson, “New Developments in Antiretroviral Drug Therapy for HIV Infection,” Chapter 4 in P. Volberding and M. A. Jacobson,
AIDS Clinical Review 1992
(New York: Marcel Dekker, 1992); B. A. Larder, K. E. Coates, and S. D. Kemp, “Zidovudine-Resistant Human Immunodeficiency Virus Selected by Passage in Cell Culture,”
Journal of Virology
6 (1991): 5232â36; and H. Mohri, M. K. Singh, W. T. W. Ching, and D. D. Ho, “Quantitation of Zidovudine-Resistant Human Immunodeficiency Virus Type 1 in the Blood of Treated and Untreated Patients,”
Proceedings of the National Academy of Sciences
90 (1993): 25â29.
New England Journal of Medicine
328 (1993): 1163â65; M. S. Hirsch and R. T. D'Aquila, “Therapy for Human Immunodeficiency Virus Infection,”
New England Journal of Medicine
328 (1993): 1686â95; V. A. Johnson, “New Developments in Antiretroviral Drug Therapy for HIV Infection,” Chapter 4 in P. Volberding and M. A. Jacobson,
AIDS Clinical Review 1992
(New York: Marcel Dekker, 1992); B. A. Larder, K. E. Coates, and S. D. Kemp, “Zidovudine-Resistant Human Immunodeficiency Virus Selected by Passage in Cell Culture,”
Journal of Virology
6 (1991): 5232â36; and H. Mohri, M. K. Singh, W. T. W. Ching, and D. D. Ho, “Quantitation of Zidovudine-Resistant Human Immunodeficiency Virus Type 1 in the Blood of Treated and Untreated Patients,”
Proceedings of the National Academy of Sciences
90 (1993): 25â29.
121
M. S. Smith, K. L. Korber, and J. S. Pagano, “Long-Term Persistence of Zidovudine Resistance Mutations in Plasma Isolates of Human Immunodeficiency Virus Type 1 Dideoxyinosine-Treated Patients Removed from Zidovudine Therapy,”
Journal of Infectious Diseases
169 (1994): 184â88; and C. P. Conlon, P. Klenerman, A. Edwards, et al., “Heterosexual Transmission of Human Immunodeficiency Virus Type 1 Variants Associated with Zidovudine Resistance,”
Journal of Infectious Diseases
169 (1994): 411â15.
Journal of Infectious Diseases
169 (1994): 184â88; and C. P. Conlon, P. Klenerman, A. Edwards, et al., “Heterosexual Transmission of Human Immunodeficiency Virus Type 1 Variants Associated with Zidovudine Resistance,”
Journal of Infectious Diseases
169 (1994): 411â15.
122
Z. Gu, Z. Gao, X. Li, et al., “Novel Mutation in the Human Immunodeficiency Virus Type
1 Reverse Transcriptase Gene That Encodes Cross-Resistance to 2',3'-Dideoxyinosine and 2'3'-Dideoxycytidine,”
Journal of Virology
66 (1992): 7128â35; and Z. Song, G. Yang, S. P. Goff, and V. R. Prasad, “Mutagenesis of the Glu-89 Residue in Human Immunodeficiency Virus Type 1 (HIV-1) and HIV-2 Reverse Transcriptase: Effects on Nucleoside Analog Resistance,”
Journal of Virology
66 (1992): 7568â71.
1 Reverse Transcriptase Gene That Encodes Cross-Resistance to 2',3'-Dideoxyinosine and 2'3'-Dideoxycytidine,”
Journal of Virology
66 (1992): 7128â35; and Z. Song, G. Yang, S. P. Goff, and V. R. Prasad, “Mutagenesis of the Glu-89 Residue in Human Immunodeficiency Virus Type 1 (HIV-1) and HIV-2 Reverse Transcriptase: Effects on Nucleoside Analog Resistance,”
Journal of Virology
66 (1992): 7568â71.
123
National Institutes of Allergy and Infectious Diseases, State-of-the-Art Conference on Antiretroviral Therapy, Bethesda, MD, June 23â25, 1993.
124
F. G. Hayden, R. B. Belshe, R. D. Clover, et al., “Emergence and Apparent Transmission of Rimantadine-Resistant Influenza A Virus in Families,”
New England Journal of Medicine
321 (1989): 1696â1702; and R. B. Belshe, M. H. Smith, C. B. Hall, et al., “Genetic Basis of Resistance to Rimantadine Emerging During Treatment of Influenza Virus Infection,”
Journal of Virology
62 (1988): 1508â12.
New England Journal of Medicine
321 (1989): 1696â1702; and R. B. Belshe, M. H. Smith, C. B. Hall, et al., “Genetic Basis of Resistance to Rimantadine Emerging During Treatment of Influenza Virus Infection,”
Journal of Virology
62 (1988): 1508â12.
125
Doctors relied almost entirely upon drug companies, directly or indirectly, for advice about use of antibiotics. The companies spent $11 billion a year in the United States alone promoting use of their products. For busy physicians who hadn't the time to sift through medical literature to learn of contrary evidence, it was hard to resist the alluring pull of pharmaceutical promotions. See R. L. Woosley, “A Prescription for Better Prescriptions,”
Issues in Science and Technology
(Spring 1994): 59â66.
Issues in Science and Technology
(Spring 1994): 59â66.
126
World Bank,
World Development Report 1993: Investing in Health
(New York: Oxford University Press, 1993).
World Development Report 1993: Investing in Health
(New York: Oxford University Press, 1993).
127
A. J. Slater, “Antibiotic Resistance in the Tropics,”
Transactions of the Royal Society of Tropical Medicine and Hygiene
83 (1989): 45â48.
Transactions of the Royal Society of Tropical Medicine and Hygiene
83 (1989): 45â48.
128
A. Chetley, “Bangladesh Drug Policy Hanging in the Balance,”
Lancet
343 (1994): 967.
Lancet
343 (1994): 967.
129
For analyses of drug development policies and politico-economic conflict, see D. E. Bell and M. R. Reich,
Health
,
Nutrition, and Economic Crises: Approaches to Policy in the Third World
(Dover, MA: Auburn House. 1988); Pan American Health Organization,
Policies for the Production and Marketing of Essential Drugs
(Washington, D.C., 1984); M. R. Reich, “Essential Drugs: Economics and Politics in International Health,”
Health Policy
8 (1987): 39â57; and World Bank,
Financing Health Services in Developing Countries: An Agenda for Reform
(Washington, D.C.: World Bank, 1987).
Health
,
Nutrition, and Economic Crises: Approaches to Policy in the Third World
(Dover, MA: Auburn House. 1988); Pan American Health Organization,
Policies for the Production and Marketing of Essential Drugs
(Washington, D.C., 1984); M. R. Reich, “Essential Drugs: Economics and Politics in International Health,”
Health Policy
8 (1987): 39â57; and World Bank,
Financing Health Services in Developing Countries: An Agenda for Reform
(Washington, D.C.: World Bank, 1987).
Other books
Con Job by Laura VanArendonk Baugh
The Prince Charles Letters by David Stubbs
The Boston Stranglers by Susan Kelly
A Shiver of Wonder by Daniel Kelley
Eagles at War by Ben Kane
The Yoga Store Murder by Dan Morse
Fireweed by Jill Paton Walsh
Yesterday's Kings by Angus Wells
You Took My Heart by Elizabeth Hoy
Unforgettable by Ted Stetson
