Read What is Life?:How chemistry becomes biology Online
Authors: Addy Pross
What is Life?:How chemistry becomes biology (22 page)

Of course any theory is only as useful as the range of phenomena it can explain. In the following pages we will revisit the life characteristics that we discussed in
chapter 1
—its complexity, its teleonomic character, dynamic character, its diversity, its far-from-equilibrium state, and its chiral character, to see how the theory of life we have offered can explain these properties. Finally, as the scientific method requires, I will make some predictions that flow directly from the theory of life that has been outlined.
Life’s extraordinary, almost incomprehensible complexity was described in
chapter 1
and we can now see that understanding the nature of DKS explains that extraordinary complexity. And as we have already discussed, the mechanism by which nature enhances DKS is through complexification—not complexification in the sense of aggregation, which we routinely see in the ‘regular’ chemical world, but one that is quite different, and is unique to the replicative world. When materials aggregate in the ‘regular’ chemical world—for example water freezing into ice or any solid crystallizing out of solution—that process happens because the solid aggregate is the more stable form. But that stability kind is thermodynamic stability, the stability kind associated with being
less
reactive, the kind that we are so familiar with in chemistry. All the physical aggregates that we generally see in the world around us derive from that simple idea—the molecules that make up those aggregates attract one another resulting in aggregates that are more stable, and hence less reactive, than the separated molecules.
But in the replicative world the stability kind that is applicable is DKS, so the aggregation pattern that is observed is the one that enhances
that
stability kind, not thermodynamic stability. And while that aggregation process will almost certainly have thermodynamic contributions to it, those contributions are secondary, and merely facilitate the primary one, which directs toward enhanced DKS. We met that interaction at its very simplest level when we discussed Gerald Joyce’s striking RNA experiment in which two
RNA molecules catalysed each other’s formation, thereby leading to the establishment of a small replicating network. In simplest terms, once a simple and relatively fragile (meaning unstable in DKS terms) entity comes about, it will tend to complexify in order to enhance its DKS. It is that Woody Allen ‘whatever works’ rule in operation again. The process occurs step by step, each step leading to a slightly more complex entity capable of enhanced replicative ability. As we noted earlier, that early process would have most likely consisted of an expanding chemical network of reactions whose overall character would be replicative—a replicating network. One can only speculate as to the specific steps that took place along the long road to early life, but the drive toward greater DKS through the mechanism of increasing complexity would characterize the process. So the above analysis couched in DKS terms explains why stability in the replicative and ‘regular’ chemical worlds are distinct, and why the aggregation processes in each of the two worlds, in particular during the process of life’s emergence, necessarily follow different paths. After several billion years of evolution the end product can be understood—replicators whose complexity is one of staggering proportions, even in simplest life, and also of extraordinary stability (in DKS terms). High complexity and high DKS go hand in hand.
As a final point, and as already noted earlier, in some instances a process of simplification, rather than one of complexification, is observed during evolution, and at both chemical and biological levels. It is that ‘whatever works’ idea again—in biology there are few hard and fast rules. Nature has no objection to taking an evolutionary step of simplification, if such a step enhances a replicator’s DKS. Whatever works! It is the DKS maximization principle
that enables evolutionary processes at both chemical and biological levels to be understood.
We have already noted that all living things are unstable in a thermodynamic sense, like a bird constantly flapping its wings to maintain its airborne state. And just like that hovering bird, all living things must constantly consume energy to maintain that far-from-equilibrium state. Yet, somehow the world is totally overwhelmed with these thermodynamically unstable entities. How come? Shouldn’t unstable things gradually disappear, rather than continue to be formed and take root in just about every feasible ecological niche? But, based on our discussion in
chapter 4
, all living things actually
are
stable, but their stability is of that ‘other kind’—DKS, the stability of things that are good at making more of themselves. As already stated, in the world of replicators the stability that matters is DKS and not thermodynamic stability. And why is it that those entities that are stable in a DKS sense are invariably
unstable
in a thermodynamic sense? Simply, because DKS depends on the system continually reacting in order to replicate, to make more of itself, and that actually requires the system to be reactive, to be unstable. Thermodynamically stable entities don’t react. They are like balls at the bottom of a slope—they have nowhere lower to roll. In other words for a living system to be a highly successful replicator it has to be DKS
stable
and thermodynamically
unstable.
We discussed how these two seemingly contradictory requirements can be simultaneously accommodated when a replicating system acquired an energy-gathering capability through a process of kinetic selection. Replicators that have an energy-gathering capability
are better replicators than those that don’t—just like cars
with
an engine are more useful forms of transport than cars
without.
Once a replicator with an energy-gathering capability came about by some chance mutation, being of higher DKS (a more effective replicator) it quickly drove its predecessor into extinction. That’s why all living systems, with no exception, have an integrated energy-gathering system in place—the photosynthetic one in the case of plants and certain bacteria, and the Krebs (citric acid) cycle for the catabolic breakdown of organic matter in the case of animals. The result: the world is full of DKS
stable,
but thermodynamically
unstable,
replicating systems. These two stability kinds, potentially in opposition to one another, can live together harmoniously thanks to that energy-gathering capability. Recently Robert Pascal, an innovative French chemist, has begun to explore the kinds of chemical processes that would have facilitated the emergence of early metabolic systems, during the transition to modern metabolic pathways.
62
One of life’s striking characteristics is its dynamic nature. We commented earlier how within the space of some months you are no longer who you were. Materially you are now largely composed of new stuff—a new you! Your blood cells, billions of them, are replaced daily, your skin cells continually turn over, the protein molecules that do most of the work in getting on with life are all continually being degraded and regenerated in a never-ending dynamic process. But how can this ephemeral and dynamic nature of living systems be explained? In fact, this particular aspect of life is one of the easiest to understand. Recall our analogy of a replicating population and a water fountain. The fountain is stable (persistent)
even though the water that makes up that fountain is turning over continuously. Different water, same fountain. For any replicating entity the same proposition holds. Because the replication reaction is unsustainable, regardless of what it is that is being replicated, a replicating system that achieves stability would be one in which the rate of replicator generation and decay would be in rough balance, one in which a steady state is established. This would be true of molecules, microbes, or monkeys, or any other replicating entity one would care to mention. In other words it is the population that is stable, with the individual entities that make up that population constantly turning over. And this continual turnover holds at all levels of complexity—molecules within cells are constantly turning over, cells within organisms are constantly turning over, and, of course, all organisms are constantly turning over. That simple fact clarifies the role that death plays in the life process. In a 2005 commencement speech to Stanford graduates, Steve Jobs, the hitech innovator said:
No one wants to die. Even people who want to go to heaven don’t want to die to get there. And yet death is the destination we all share. No one has ever escaped it. And that is as it should be, because Death is very likely the single best invention of Life. It is Life’s change agent. It clears out the old to make way for the new. Right now the new is you, but someday not too long from now, you will gradually become the old and be cleared away. Sorry to be so dramatic, but it is quite true.
Death then is not just something bad that happens to us living things. Death is part of the life strategy. Seeking eternal life? The term is an oxymoron. There can be no eternal life because the very basis of life is its transient and dynamic nature.
Though Darwinian theory was able to relate all living things to one another, the source of life’s spectacular diversity remains unresolved. As we discussed in
chapter 1
, Darwin himself remained uncertain on this key point. In his
Origin of Species
Darwin did propose a Principle of Divergence, but whether that principle was independent of his principle of natural selection, or derived from the principle, was left open and, interestingly, the issue continues to preoccupy modern biologists. However, the theory of life that we have described, based on the DKS concept, seems to offer some resolution of this issue. It turns out that the key to understanding life’s extraordinary diversity lies in the
topologies
of the two chemical worlds—the ‘regular’ and replicative worlds, and the difference between them. Let me explain.
I have already explained that all chemical systems are directed toward their most stable form. That means that different chemical systems that are composed of the same elements will all want to end up at the same place, just like different balls rolling down a hilly terrain from different locations on that terrain will all want to end up in the same location—the lowest point in the valley below. If you take any mixture of hydrocarbons—that’s just a chain of carbon atoms joined to hydrogen atoms, such as we find in gasoline—and react that mixture with oxygen in what is called a combustion reaction, the resultant product is carbon dioxide and water. It doesn’t matter which hydrocarbon you start with, you always end up with carbon dioxide and water, because that is the most stable form of a mixture of C, H, and O atoms. All hydrocarbon-oxygen mixtures converge to carbon dioxide and water. That argument may be generalized to chemical systems as a whole, so one could say that the grid that connects the world of ‘regular’ chemical substances is
convergent,
as illustrated schematically in Fig. 7a. All roads lead to Rome and all chemical reactions are directed to what is called their thermodynamic sink—the lowest energy possibility for that combination of atoms. That’s how a chemist can frequently predict the result of a chemical reaction, that’s how he/she knows where the chemical system ‘wants to go’.
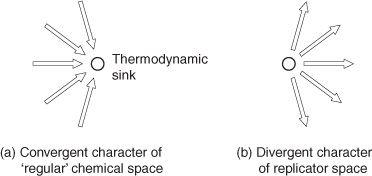
Fig. 7.
Schematic representation of branching patterns within ‘regular’ chemical space (convergent), and within replicator space (divergent).
But let us now turn to the world of replicating systems. In contrast to a ‘regular’ chemical system, which may be thought of as contained, or closed, a replicating system must remain open at all times to allow the replicating reaction to proceed unimpeded. Being open means that building blocks for replication, as well as the energy to support the replication process, must be continually provided. In other words, in comparison to a ‘regular’ chemical reaction, which may be carried out in a closed container, a replicating reaction must remain open to the surroundings. That different situation results in the path to greater DKS being
divergent,
as illustrated in Fig. 7b, rather than convergent. Why? Because the
path forward to greater DKS will depend on what’s available at that time and in that place, and any number of different paths toward more stable systems (in a DKS sense) are, in principle, feasible. Some replicator X might pair up with some molecule Y to create a more stable X/Y system compared to X on its own, but it also might pair up with some other molecule Z, thereby creating a stable X/Z system. The possibility of different complexification pathways leads to diversification. All stable replicating systems are continually replicating, occasionally mutating, continually complexifying, thereby exploring the world of replicating systems for increasingly effective replicators. The topology of the world of replicating systems is inherently divergent.

