Why Is Milk White? (18 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

When the sun is near the horizon, there is more air between it and your eyes. The light near the sun has more red and yellow because light that is scattered only one or two times does not change direction much. There is also more dust and smog, which scatter more red and yellow light. So sunsets are red, yellow, orange, and pink.
Water is clear because nothing in it reflects light.
You can see things because light from them gets into your eyes. If something has nothing in it to bend or bounce light, you don't see it. This is why air and glass are both clear.
There are times when you can see these things. If the air has dust in it, you see the dust. Or if the air in one place is hotter or colder than in another place, you can see the effect of the difference because it bends the light, and things on the other side look distorted or appear to move.
If glass is perfectly clean, you might not know it is there and walk into it. But if the glass is not smooth and flat, you can see how it distorts things you see through it, and you can tell where it is. If the water or glass has a dye in it that absorbs some color of light, then you see all the colors but that one, and you know the glass is there. If the glass absorbs yellow light, it will look blue.
Water and most glasses actually do absorb red and yellow light more than they do blue, so if there is enough water or glass, it looks blue.
Grass is green because you water the lawn. Grasses that have evolved to tolerate drought have less chlorophyll than grasses in wetter environments. Chlorophyll (the pigment that absorbs red and blue light to make sugar from water and carbon dioxide) reflects green light, but in absorbing the other colors, it warms up.
If there isn't enough water, the blades of grass close up the little holes, called
stomata,
where water evaporates to cool the leaves. If there is not enough water, the plant produces less of the chlorophyll molecule and can survive the heat better.
In the summer, many grasses (especially in arid or semi-arid areas) lose their chlorophyll altogether. They turn the color of straw. In some the chlorophyll is lost because the sunlight breaks it down, and the plant does not replace it. In others, the plant actively removes the chlorophyll and stores the valuable materials
in another part of the plant. In some annual grasses, that part is the seeds.
We can color the milk by either adding something or taking something away. The white color of milk is caused by particles and droplets in the milk that reflect (scatter) the light that hits them. If you remove the droplets of fat from whole milk, it takes on the slightly bluish tint of nonfat milk.
If you add brown powdered cocoa, the cocoa particles absorb a good deal of the light that hits them, but they reflect enough red and yellow to appear brown. The milk adds white reflections, so the result is a lighter shade of brown than that of the original cocoa powder.
You can make pink milk by adding red food coloring. The red dye absorbs green and blue light. The milk adds white, giving us pink.
Because of the effect the droplets of fat have on the light, it is hard to get deep, dark colors when adding dyes to milk. Even adding India ink only makes a gray color.
Carbon dioxide. A common type of disappearing ink is based on an acid-base indicator called
thymolphthalein.
This is a molecule that absorbs visible light when it is in an alkaline solution and becomes colorless in a neutral or acidic solution.
To turn it into a blue ink, sodium hydroxide (lye), a strong alkali, is added. As long as the solution is basic (alkaline), it will be blue. But if you allow the ink to soak into paper or cloth, so that there is a lot of surface area in contact with the air, it will absorb carbon dioxide from the air. Carbon dioxide mixes with water to form carbonic acid (the bubbly ingredient in soda pop). The acid neutralizes the lye, forming sodium carbonate (washing soda). The neutral solution is no longer blue, but colorless.
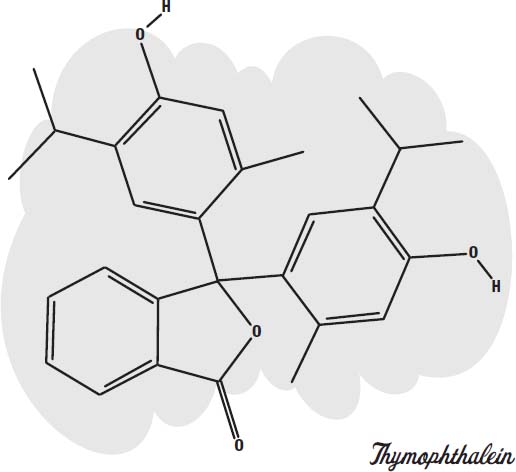
You can make it blue again by adding an alkali, such as baking soda or ammonia (or more lye).
Some milk contains a lot of carotenoid pigments in the butterfat. When the butterfat is skimmed off and churned into butter, the butter is yellow.
You make homemade butter by shaking a jar full of heavy whipping cream (or overwhipping your cream), but it is seldom as dark a yellow color as the butter you buy in the store. This is because commercial butter often has carotenoid pigments added
to make it look richer or to match the buyer's idea of what butter should look like.
You can extract your own carotenoid pigments to color your own homemade butter if you like. First, grate some carrots. Then melt some butter in a pan, and gently sauté the carrots in the butter. The melted butter will extract some of the carotenoid pigments from the carrots. The oily liquid that floats to the top is clarified butter, which is quite a bit darker than it used to be.
Cool the clarified butter. Now you can use a mixer to blend it into the homemade butter to get a deep yellow color.
There are a number of ways to whiten your teeth. Many toothpastes contain sodium percarbonate or calcium peroxide. Both of these chemicals release hydrogen peroxide when they are mixed with water. In the toothpaste they are more stable than plain hydrogen peroxide would be, so the toothpaste won't decompose before you have a chance to use it.
Your teeth can be yellow for several reasons. Many of the foods you eat can stain teeth. Coffee, tea, and tobacco are famous for yellowing teeth, but many other foods also have this ability. Peroxide toothpastes, or a rinse of 3 percent hydrogen peroxide, can help remove most of the stains. Peroxide bleaches the yellow by oxidizing the stain molecules so that they no longer absorb light.
Bacteria that live in your mouth make a protein film on the teeth called a biofilm. This is easily stained and may be slightly yellow all by itself. Peroxide bleaches the stain molecules, but it also breaks up the protein film so you can brush it away.
If you have been brushing your teeth for many years, the white enamel on the outside of the tooth may get thin enough that the yellow dentin inside the tooth shows through. Peroxide can penetrate into the dentin and bleach the yellow to a whiter color.
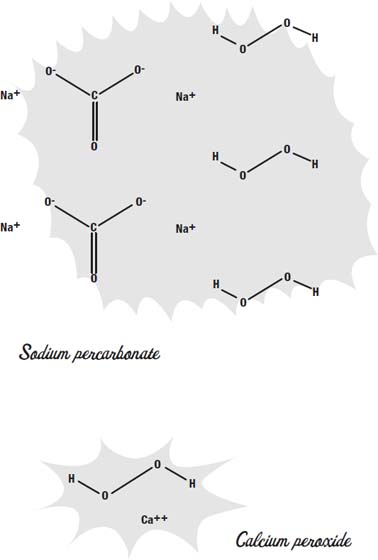
There are many kinds of bleach. Each of them works in a slightly different way to do the same thing: change a molecule that absorbs visible light into a molecule that does not.
If we look at a molecule of cyanidin (an anthocyanin dye molecule that gives some flowers their color), we can see the pattern of alternating double and single bonds that allow the molecule to absorb visible light.
The electrons in these bonds are actually shared, or
delocalized,
across all of the atoms in the rings, which is why they can slosh around the molecule like water in a bathtub. When they slosh at the same frequency as visible light, they can absorb it.
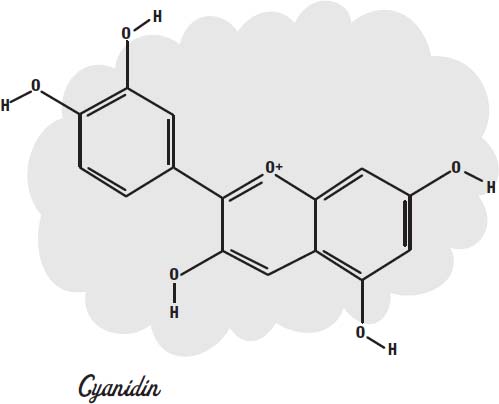
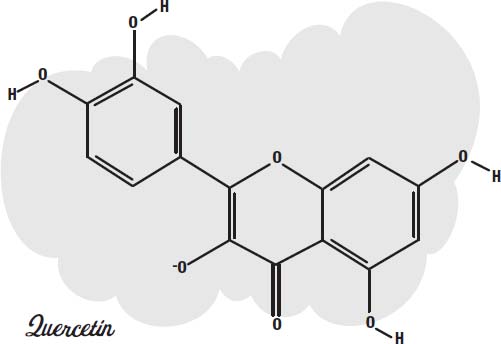
Chlorine bleach and hydrogen peroxide both provide oxygen atoms that can combine with the molecule to change the loops so they no longer have delocalized electrons to slosh around.
Quercetin is a molecule in which oxygen atoms have been added to cyanidin. The ring in the middle no longer has delocalized electrons, and the molecule is colorless.
Other chemicals can also bleach dye molecules such as cyanidin. Sulfur dioxide and sulfites can add SO
2
groups to the molecule that also steal electrons from the center ring, causing the molecule to become colorless.
COLOR BLOSSOMS
Materials
| Small bowl | Food coloring |
| Whole milk | Liquid dishwashing detergent |
My last chemistry book,
Culinary Reactions,
deals with chemistry from the viewpoint of the kitchen and discusses food chemistry in some detail.
One of the subjects in the book is how proteins and fats interact in water. A good example of this is how proteins form around droplets of butterfat in cream and whole milk, stabilizing the emulsion of fat in water.
You can destabilize the emulsion by adding something that combines with fat and water better than the proteins do. Soaps and detergents do exactly that, and you can demonstrate their effect with a very colorful display.
In the photo on the next page, I started off with a bowl of whole milk (nonfat milk won't work), to which I added some drops of food coloring. Notice that the drops are just sitting there, not mixing much at all with the milk.
Next I added a drop of dishwashing detergent.
The colors exploded, racing away from the detergent. But they didn't stop. They continued to mix and swirl around the bowl for more than a minute. They flowed and folded and moved around, quite unlike what they were doing before I added the detergent.
What happened is that the detergent interacted with the oil, the water, and the proteins in the milk. The detergent had molecules on which one end liked to stay in water and the
other end liked to stay in fats and oils. Many of the proteins in the milk also had parts that were water-loving (
hydrophilic
) and other parts that were wateravoiding (
hydrophobic
).

See the video at
http://youtu.be/SwsCQtipAus
The detergent moved in to replace the proteins at the interface between the butterfat and the water. But the detergent also attached to the proteins at their water-loving and water- avoiding parts, and this changed the shapes of the proteins and changed how the proteins attached to one another.
All of this rearranging can take some timeâup to several minutesâto complete. As the molecules rearranged, they pushed the water and the food coloring around, causing them to stir up into beautiful blossoms of color.
