Why Is Milk White? (24 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

Most forms of radiation humans encounter are harmless or even necessary for life. Without light and heat, there would be no life. We use radio waves for communication, and they pass through us without harm.
In medicine, radiation usually refers to
ionizing radiation.
This is radiation that has enough energy to strip electrons away from atoms, forming ions. Since electrons are what form the bonds between molecules in our bodies, ionizing radiation can change those bonds in harmful ways, creating burns or damaging DNA, which can cause cancer.
Radioactivity
refers to something that happens to atoms that have too many or too few neutrons in them in proportion to the number of protons. These atoms are unstable and decay into more stable atoms by emitting radiation.
You read in the last question how radioactive carbon-14 decayed into nitrogen; it does so by changing a neutron into a proton and an electron (the latter of which speeds away as a beta particle). More famously, uranium goes through a series of radioactive decays into various elements, eventually turning into lead.
Radioactive elements produce many types of radiation as they decay. They can emit electrons, alpha particles, neutrons, neutrinos, and gamma rays (a form of high-energy X-rays). They can also produce larger atomic nuclei that fly away as the atom splits.
Actually, chemists extract the jelly from petroleum. Petroleum jelly is a mixture of hydrocarbon chains with lengths of 25 or more carbons. A hydrocarbon is just what it sounds like, a chain of carbons on which every carbon has hydrogens bound to it.
The length of the chains determines whether the hydrocarbon will be a liquid, like gasoline (with lengths from 6 to 10 carbons) or mineral oil, or a solid, like paraffin wax (with 20 to 40 carbons in the chain). Petroleum jelly is in between, with around 25 carbons (there are a number of different molecules of different lengths in the mixture).
A crude black form of petroleum jelly was originally discovered by workers on oil rigs, as a gunk that fouled up the machinery. A chemist named Robert Chesebrough refined this into a
white paste that has no color, odor, or taste and marketed it as Vaseline in 1872.
As a grease that prevents skin from drying out and keeps bacteria away from burns and wounds, Vaseline found many uses in the home medicine cabinet. It melts at a temperature close to human body temperature, so it becomes a near-liquid when applied to the skin.
You may know Chesebrough's name through the brand Chesebrough-Ponds, now a subsidiary of Unilever. The brand includes Ponds Cold Cream and Cutex nail polish remover.
Chemicals foam up because they release gas. The most familiar foaming reaction is probably that of baking soda and vinegar. A similar reaction is what makes pancake batter rise. Sodium bicarbonate is a salt of a strong base (sodium hydroxide) and a weak acid (carbonic acid). When you add an acid to a salt of another acid, you generally release a gas that makes the second acid when mixed with water. Baking soda releases carbon dioxide gas, which makes carbonic acid when mixed with water. You have swallowed carbonic acidâit is the bubbly water to which sugar and flavorings are added to make flavored sodas.
Another familiar reaction that produces bubbles of gas is that of hydrogen peroxide when it meets a catalyst that breaks it down into water and oxygen. That catalyst is a protein called
catalase
that most living things produce, because hydrogen peroxide is a harmful byproduct of metabolism, and the catalase breaks it down.
Reacting any of several metals (such as aluminum, magnesium, or zinc) with an acid will release hydrogen gas. The metal replaces the hydrogen in the acid, leaving a salt (such as aluminum chloride if the acid was hydrochloric acid) and bubbles of hydrogen.
Electricity can create bubbles of gas. In water, electricity will release hydrogen bubbles from one electrode and oxygen from the other. In salt water, chlorine gas is released instead of oxygen.
To make bubbles into a foam, it helps to have some molecules that reduce the surface tension of in the liquid the bubbles are in. Soap and detergents will do this. By lowering the surface tension, the soap stabilizes the thin layer of water around the bubble in the foam. Something called the
Marangoni effect
says that water will flow into the area that has the lowest surface tension. As the bubble stretches, the concentration of soap is reduced. This causes water to rush in to strengthen the thinnest layers in the bubble.
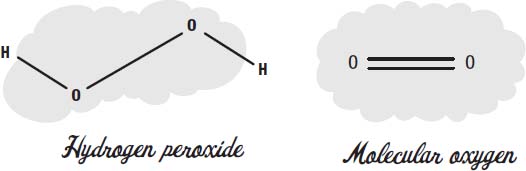
Hydrogen peroxide is two atoms of oxygen, each attached to a hydrogen atom. You can think of it as a water molecule with an extra oxygen in the middle.
The oxygen we breathe has two oxygen atoms in itâwe call it molecular oxygen. In molecular oxygen, the two atoms share two electrons, in what we call a double bond.
When two oxygens are connected with a single bond, we call it a peroxide. Hydrogen peroxide is the simplest example of a peroxide molecule.
Pure hydrogen peroxide is a liquid a little heavier and thicker (more viscous) than water. It is a strong oxidizer and can start fires if it contacts flammable materials like wood or paper.
In the home, dilute solutions of hydrogen peroxide are used to disinfect and bleach. Three percent hydrogen peroxide is used to
clean wounds and as a mouth rinse to kill bacteria. Stronger solutions are used to bleach hair.
Cells produce hydrogen peroxide as a byproduct of metabolism. But hydrogen peroxide is also used by the immune system as a signal that attracts white blood cells to an infection.
A 3 percent solution of hydrogen peroxide will produce ten times its volume in oxygen if a catalyst (such as blood or dry yeast) is added. Most common metals will also act as a catalyst for the dissociation of hydrogen peroxide into oxygen and water. Adding a catalyst to hydrogen peroxide is thus an easy way to generate oxygen.
These days, soda cans are usually made of an
alloy
, or metallic mixture, consisting mostly of aluminum with about 1 percent manganese and 1 percent magnesium. The extra metals give the aluminum more strength and make it easier to form. Other metals are in the alloy in much smaller quantities, including copper, gallium, iron, silicon, titanium, vanadium, and zinc. Some of these are added deliberately to improve the alloy, while others are present only because they would be difficult to remove from the raw materials used to make the alloy.
The lid of a soda can is made from a different alloy, which contains up to 5 percent magnesium. This alloy is more expensive and not as strong as the alloy used for the body of the can, but it is more easily formed into the complex easy-open top. This is why the top of the can is so much smaller in diameter than the rest of the canâit makes it cheaper, and a smaller lid is stronger, so the weaker, more expensive alloy is not a problem. The bottom of the can is also made smaller in diameter so that the cans will stack.
Soda cans are lacquered with plastic, both to prevent the acids in the contents from attacking the aluminum and to keep the outside of the can from reacting with things it contacts in shipping and
storage. Food-grade petroleum jelly and food grade waxes are used as lubricants when the cans are constructed and crimped closed.
Soda cans are designed to hold a maximum pressure of 95 pounds per square inch. This is much more than the contents of the can are usually under and provides some margin for heat and shock in shipping.
The two temperature scales were developed by two different people, and they use two different ideas of where to put the zero and 100 marks.
Daniel Fahrenheit based his original temperature scale on three temperatures. The coldest he had availableâice in a brine made from salt and ammonium chlorideâwould be the zero point (0°). He wanted human body temperature to be near 100°. His third temperature was the melting point of ice. To make it easier to mark his thermometers, he made the freezing point 32° and the human temperature 96°, so he could make the markings by simply cutting the interval between 96° and 32° in half six times.
Later, the scale was adjusted so that water boiled at 212° and froze at 32°. This made human body temperature closer to 98°. The interval between freezing and boiling was 180°, an easy number to divide into parts.
In 1744 a different scale was created, based on one made by Anders Celsius a couple years earlier. His scale used the freezing point of water as 100° and the boiling point of water at sea level as 0°. After his death, the scale was reversed so that higher temperatures had higher numbers.
Because a degree was 1/100 of the difference between melting and boiling, it was called the
centigrade scale
for over 200 years. But that term was also used for other measurements, and the international standards bureau changed the name to Celsius in 1948.
Concrete, steel, and geometry. There are many kinds of bridges, and as many ways for them to be strong.
Suspension bridges, such as the Golden Gate Bridge in San Francisco, get their strength from steel and geometry. The bridge is mostly in tension: the forces on it try to pull it apart more than they try to compress it. Steel has very high strength under tension, so steel cables are used to build the bridge. The towers supporting the bridge are under compression and are made of steel, sitting on enormous concrete piers.
Other bridges are often made using arches of concrete. Concrete has very high compression strength, and an arch is almost entirely under compression instead of tension. This is due to the geometry of the arch, which channels the load downward into the ground.
Most smaller bridges, such as those used for freeway overpasses, use a trick that combines the tensile strength of steel with the compression strength of concrete. Reinforced and pre-stressed concrete are building techniques where steel reinforcements are added to concrete where it will be under tension. Little or no reinforcing is needed where the structure is only under compression forces. In a bridge span, the steel will be on the bottom and the concrete on the top. As the bridge is loaded, the concrete compresses, and the steel pulls.
Pre-stressed concrete takes this one step further by putting the steel parts under tension before the concrete is poured.
Water is a polar molecule, with one end positively charged and the other negatively charged. These charges interact with ionic bonds in substances like table salt. The water molecules surround the charged particles in the salt with oppositely charged ends of the
water molecules. This reduces the effective charges on the sodium and chlorine ions, so they no longer attract one another as much as they did before.
The rest of the work of dissolving the salt is done by heat and by the motion of the water, both of which jostle the ions apart, so they can be surrounded by water molecules and isolated.
The polar nature of water is also why nonpolar molecules do not dissolve in water. Substances like oils, fats, and waxes have no charged parts to attract water molecules. The water molecules are attracted to one another by their charges, and they leave the nonpolar molecules alone. This pulling together of all the water molecules acts to pull them away from uncharged molecules, so oil and water don't mix.
Since water is denser than most oils, fats, and waxes, it falls to the bottom of the container, and the oil is left behind, on top.
Other materials, like metals, sand, rocks, or plastic, are also unaffected by water, and for the same reasons, only in reverse. The molecules in rocks and metals attract one another much more than they do water. So the metal atoms stick together and leave the water behind. Since they are generally heavier, they sink to the bottom, leaving the water on top.
Plastic is long chains of small molecules called
monomers
that are joined together. The long chains are called
polymers.
Your hair, your fingernails, and the tendons in your arms and legs are made of polymers. In those polymers (called
proteins)
the building blocks are called
amino acids.
They are chained together to form strong ropes of material to be used where strength is important.
You can make your own protein plastic from milk. Add some vinegar to nonfat milk, and it will curdle into white clumps of casein protein. You can compress this into a mold and let it dry into a hard plastic. Umbrella handles used to be made this way.
EXTRACTING IODINE
Adult
supervision
required
Materials
Test tube with stopper or similar glass container, such as a small glass jar with lid
Tincture of iodine (available at drugstores and pharmacies)
Water
Toluene solvent (available at hardware stores) or naphtha cigarette lighter fluid (available at drugstores)
Eyedropper or pipette
Glass jar with lid
Hair dryer with no-heat setting
One of the projects coming later uses crystals of the element iodine.
For someone like me, finding iodine crystals is as simple as reaching up to the top shelf in the lab. But for most of the readers of this book, the pure element is not so easy to come by.
However, there is a tiny bit of iodine in that bottle of tincture of iodine you can find at the pharmacy. There is about half a gram in each fluid ounce of the disinfectant.
Extracting the pure element is simple and demonstrates how clever chemists can be using simple supplies and materials. The principle is called
partitioning,
or sometimes
liquid-liquid extraction.
It takes advantage of the fact that some things dissolve more easily in one liquid than another.

The liquid in tincture of iodine is alcohol. Iodine does not dissolve well in alcohol, and dissolves even less well in water. To get the elemental iodine to dissolve in either of these solvents, a little potassium iodide is added. The iodide ion helps more of the pure iodine dissolve.

Pour a small amount of the tincture of iodine into a test tube or a similar glass container with a stopper or lid, so it's no more than about one-sixth of the way full.
(Do not use a plastic container,
because the toluene we'll be adding in the next step dissolves plastic.) Then add an equal amount of water and an equal amount of toluene (available in stores either as a pure solvent
or as naphtha cigarette lighter fluid). The water dilutes the alcohol and makes the iodine less soluble in the mixture. The toluene will float to the top, since it does not mix well with either alcohol or water.

Iodine dissolves easily in toluene. In the photo on the previous page you can see the bottom layer of 50 percent water and 50 percent tincture of iodine, and above it the layer of toluene, which has already started to get pinkish-violet from the iodine it has absorbed.
The next step is to stopper the test tube (or close the glass jar, etc.) and shake well for 15 to 20 seconds. The two layers will mix, and there will be some froth at the top, but when you stop shaking, the two layers will fairly quickly separate again. After 30 seconds of sitting still, the top layer of toluene can be seen stained a dark purple by the iodine it has extracted from the tincture, as in the photo above.

The next step is to use an eyedropper or a pipette to carefully remove as much of the top layer of dark purple toluene as you can into a glass jar, without getting any of the alcohol and water mixture in with it. There will be a little toluene left in the test tube, but that is OK.
Now you have a jar with dark purple toluene in it. You need
to evaporate the toluene while losing as little of the iodine as possible. Iodine slowly vaporizes at room temperature, but if you give the toluene a lot of flowing air, it will evaporate faster than the iodine does. I used a blow dryer that had a setting that allowed the air to flow without any heat (heat would evaporate the iodine quickly). In a few minutes, the toluene had evaporated, and I had pure iodine crystals in the bottom of the jar.

Save the crystals by tightly closing the jar. You will need them for a later project, “Latent Fingerprints” (
page 202
).
