Allies and Enemies: How the World Depends on Bacteria (12 page)
Read Allies and Enemies: How the World Depends on Bacteria Online
Authors: Anne Maczulak
Tags: #Science, #Reference, #Non-Fiction

avoid typhus-ridden Rozvadow. The contrived typhus epidemic saved
almost 8,000 lives, many of them Jews.
People and their pathogens have continuously traded victories
and defeats. Sometimes the bacteria win, such as in plague and syphilis epidemics. Sometimes the guile of humans triumphs as in d’Herelle’s phage therapy. But do people ever truly defeat bacteria?
The search for the magic bullet ended when a shy microbiologist discovered the “miracle drug” penicillin, or so it seemed at the time.
This page intentionally left blank
3
“Humans defeat germs!”
(but not for long)
In bacteria, one mutated cell appears for every 100 million normal cells. Because some bacteria reproduce as quickly as every 20 minutes, new populations of mutants emerge literally overnight. Most mutations give no discernible advantages or disadvantages to the cell.
Unfavorable mutations make bacteria vulnerable to other microbes or the environment, and these cells and their genes disappear forever.
On rare occasions a mutation gives a bacterial cell a favorable characteristic called a trait that enables the bacterium to outperform others.
Most people remember from Biology class that a favorable mutation appears only because of a random event. “The survival of the fittest” comes not by plan but by luck. Chance mutations in bacterial DNA produce slight, random changes in a single gene, and this altered gene gives the cell the ability to grow faster, swim farther, absorb more nutrients, or withstand heat better than its brethren.
When this special cell divides, two identical cells appear that also outcompete others until the new gene has become part of a new, evolved population.
In 1988, John Cairns found in
E. coli
a ploy that turned the concept of randomness on its head. Cairns’s
E. coli used adaptive mutations, which occurred when a specialized mutator gene detected a stimulus in the environment. Mutator genes prompt the cell’s mutation rate to speed up, thus increasing the chance that one of E. coli ’s 4,377 genes will mutate in a favorable direction. More than 30 mutator genes have now been located in E. coli and similar genes in Pseudomonas aeruginosa, a water-associated microbe and common invader of burns and invasive devices (intravenous tubes, catheters, 63
64
allies and enemies
and so on). Are bacteria choosing how and when they mutate? If so, an
idea that once belonged only in science fiction may be a reality.
What is an antibiotic?
Antibiotic means “against life” and belongs to two groups: true antibiotics and bacteriocins. A true antibiotic is made by a microbe to kill other unrelated microbes.
Penicillium
mold produces the antibiotic penicillin to kill bacteria that venture too close to its territory. Bacteriocins come from bacteria to kill other bacteria. For example, E. coli produces the bacteriocin colicin that kills bacteria in E. coli’s family of enteric microbes. Some bacteriocins kill different strains of the very same species, all for the purpose of reducing competition for space,
food, light, and water.
An antibiotic that kills bacteria outright is a “cidal” agent, or bactericidal. Weaker antibiotics that merely slow down bacterial growth are called bacteriostatic. Penicillin is bactericidal because it prevents susceptible bacteria from building a rigid cell wall, forcing the bacteria to succumb to toxins in their environment. Tetracyclines, by contrast, interfere with protein synthesis, which may not necessarily kill the cell. The cells might switch to an alternate synthesis pathway, but this slows their reproductive rate, so tetracycline has done its job.
Figure 3.1 illustrates a simple laboratory test that determines the susceptibility of bacteria to various antibiotics.
The structure of an antibiotic includes several carbons and hydrogens plus carbon rings and branches that make the molecule look complex. Nature developed the intricate structures to make it harder for bacterial enzymes to recognize and degrade an antibiotic. But humans interfered with nature’s plan by using increasing amounts of antibiotics and thus exposing bacteria to the compounds more frequently. Twenty years after the first commercial use of penicillin, antibiotic-resistant bacteria emerged. Resistant bacteria now exist for all of the natural antibiotics in Table 3.1. Today chemists try to stay ahead of bacteria by synthesizing new antibiotic molecules with more complexities in the hope of outwitting pathogenic bacteria.
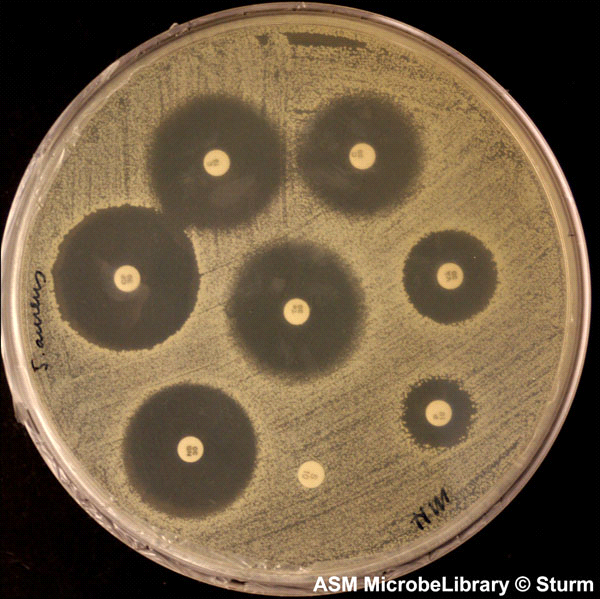
chapter 3 · “humans defeat germs!” (but not for long)
65
Figure 3.1 Kirby-Bauer antibiotic testing. Small paper discs soaked in different antibiotics cause varying levels of inhibition against bacteria. This test is a refinement of Alexander Fleming’s discovery that mold spores can kill bacteria due to the secretion of antibiotic. (Reproduced with permission of the American Society for Microbiology MicrobeLibrary, www.microbelibrary.org) Table 3.1 The main natural antibacterial antibiotics
Producer
Antibiotic
Molds
Acremonium
Cephalothin
Penicillium
Griseofulvin and penicillin
Bacteria
Bacillus
Bacitracin and polymyxin
Micromonospora
Gentamicin
Streptomyces
Amphotericin B, chloramphenicol, erythromycin, neomycin,
streptomycin, and tetracyclines
66
allies and enemies
The United States produces 25,000 tons of antibiotics annually.
Most of the drugs go to human medicine and agriculture. Cattle, hogs, sheep, goats, and poultry raised for meat receive 70 percent of the supply to promote growth rate and repel infections that travel fast through factory farms. The remainder of the antibiotic supply goes to dogs, cats, horses, and other domesticated animals, pelt animals, fish, and plants and trees.
Meat producers have suffered strident criticism for giving the animals they raise a constant intake of antibiotics. When I began my college career as an animal science major, we took for granted the benefits of antibiotic use in meat animals. Beef, pork, and poultry received subtherapeutic levels of more than one drug for no specific reason other than the possibility of increased weight gain. The mounting questions regarding this practice spurred researchers to study bacteria in the digestive tract of healthy ruminant and nonruminant animals receiving antibiotics. Antibiotic-resistant bacteria have been recovered from these animals, but it can be difficult to prove that the antibiotics led to resistance.
Food producers insisted for years that antibiotics are needed for
efficiency in meat production. Meat producers give these drugs to animals to prevent the spread of infection in a population of animals living in very close quarters from birth to slaughterhouse—this is the
reason behind the term “factory farming.” Factory farming increases
the nebulous condition we call stress when animals spend their entire lives squeezed together, and stress weakens immunity. The high density of individuals creates a higher risk for infection. Perhaps the logic behind factory farming and administering antibiotics to livestock makes no sense, and halting both would be a better choice.
The agriculture industry argues that efficient mass-production
style farming keeps food costs low. Researchers have discovered shifts
in the proportions of intestinal bacteria in antibiotic-fed animals. It has been more difficult, however, to determine the connection between altered bacterial populations and faster growth in animals.
Large-scale agriculture has been reluctant to share its antibiotic
methods, so the public will have a hard time learning which antibiotics, if any, are in the meat they buy.
chapter 3 · “humans defeat germs!” (but not for long)
67
The environmental effects of subtherapeutic antibiotics in meat
animals remain largely unknown. Two outcomes seem likely, however.
First, antibiotic-resistant bacteria shed in manure enter the environment and cause harmful consequences in ecosystems, and second, eating rare meats or runny eggs increases a person’s chance to ingest resistant bacteria. Food is not sterile and cooking does not guarantee the removal of all potential pathogens; cooking reduces bacterial numbers to safer levels. We get away with ingesting a pathogen here or there throughout the week because the dose of the microbe is lower than required to cause infection. At the same time, our native bacteria and immune system protect the body from exposure to low numbers of pathogens.
The European Union and Canada ban antibiotic use in meat animals, and the World Health Organization has taken a stance that conveys its concern over antibiotics in agriculture. The United States still uses antibiotics, and meat-producing states continue to contend that no indisputable evidence exists to prove that meat antibiotics lead to drug resistance in people. Indisputable evident is exceedingly difficult to find in any field of science, so consumers have been left with making their own decisions on the safety of meat products.
Antibiotics that escape farms in runoff from manure piles enter
surface waters. In a perfect world, the wastewater would be directed
to a wastewater treatment plant without contaminating the environment. This is impractical considering the magnitude of daily manure output in the United States alone. Wastewater treatment and drinking water disinfection provide poor protection against antibiotics. In 2005, researchers from the University of Wisconsin detected six antibiotics in treated wastewater:
·
Tetracycline
—For skin, urinary tract, and some sexually
transmitted diseases
·
Trimethoprim
—Childhood ear infections, urinary tract
infections
·
Sulfamethoxazole
—Used in combination with trimethoprim
for treating ear, bronchial, or urinary tract infections
·
Erythromycin
—Treats respiratory tract infections
68
allies and enemies
·
Ciprofloxacin
—Lower respiratory, urinary, and other
infections
·
Sulfamethazin
—
—
For respiratory and other infections in animals
I am not suggesting that treated water is a source of danger in every community or that antibiotics in water definitely cause harm.
The drugs in the study described here had, furthermore, been
detected in parts per billion levels, equivalent to one corn kernel in a nine-foot silo of corn.
Drugs entering the environment year after year are affecting
ecosystems, but scientists do not yet know all the details. Therefore,
the public has no way of knowing. But imagine an antibiotic injected
into a sick horse ending up miles away in a glass of tap water or a plate of oysters Rockefeller.
Antibiotics made immediate and profound effects on human
health when they first became available, and few people foresaw trouble. Trouble would come, and the first warning came from a sur—
prising source. Unfortunately, the world missed the message concerning antibiotic resistance until it was too late.
Inventing drugs is like making sausage
In 1897, a 23-year-old doctoral student submitted his graduation thesis to the Institut Pasteur, alluding to a new drug that might be helpful in fighting bacterial infections. The document described a Penicillium mold that killed E. coli in Petri dishes and cured laboratory animals injected with live typhoid bacteria. The reviewing faculty found Ernest Duchesne’s work uninspired, but they granted Duchesne a diploma along with little encouragement for a career in science. He enlisted in the French army. Before leaving, Duchesne discarded his laboratory notes; his thesis disappeared into a corner of the institute.
