Allies and Enemies: How the World Depends on Bacteria (28 page)
Read Allies and Enemies: How the World Depends on Bacteria Online
Authors: Anne Maczulak
Tags: #Science, #Reference, #Non-Fiction

tenfold when electrons travel through the film. Perhaps energy companies will one day feed sugar and oxygen to massive biofilm fields and thus produce electricity as well as clean water, a by-product of photosynthesis. Algae and cyanobacteria both possess the capabilities to do this. Biotechnology could also engineer the microbes to produce hydrogen or ethanol.
The waste problem
Bacteria degrade pollutants in soil, surface waters, and groundwaters.
These pollutants include pesticides, vehicle and jet fuels, paints, organic solvents, and buried ammunition. Bioremediation scientists take genes from bacteria that metabolize these pollutants and insert the genes into bacteria that grow faster in nature. Bioremediation laboratories now have collections of bacteria that degrade chemical pollutants, detoxify metals such as mercury, or decompose radioactive
compounds. Specialized bioreactors can grow biofilms on their interior surfaces (see Figure 7.1) and remove pollutants from water as it flows through the vessel. Bioremediation also seeks the bacteria that


156
allies and enemies
live in the acid drainage from ore and coal mines and which trickles
24/7 into more than 10,000 miles of U.S. rivers and streams. The traits enabling the bacteria to thrive in these places make them perfect gene donors for making bioengineered bacteria for mining pollution cleanup.
Bacteria
Metal
(a)
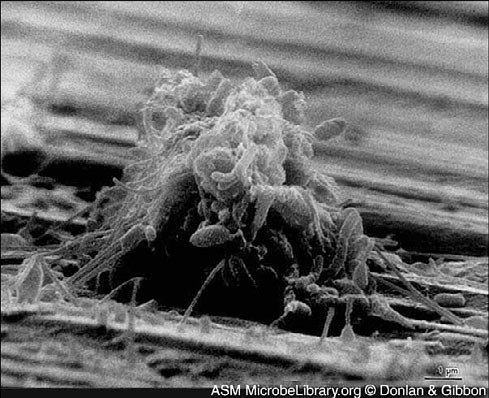
(b)
Figure 7.1 Community formation. In this series of photos, bacteria colonize the metal surface of a cooling system condenser, a process called fouling.
(a) Scattered bacteria adhere to a copper-nickel surface; (b) cells and extracellular materials accumulate;

chapter 7 · climate, bacteria, and a barrel of oil
157
(c)
Bacterial
filament
Diatom
(d)
Figure 7.1 (c) filaments extend and trap more cells; and (d) bacteria, freshwater diatoms (type of algae), corrosion products, and clay particles imbed in intertwining filaments. (Reproduced with permission of the American Society for Microbiology MicrobeLibrary (http://www.microbelibrary.org))
158
allies and enemies
Wastewater treatment plants rely on mixtures of aerobic bacteria
to degrade the substances in the incoming water. This step happens
in the plant’s large outdoor pools filled with dark liquid. Wastewater
plants mix the suspension with big paddles and constantly bubble air
through the water to keep the bacteria growing. Treated wastewater
gets a dose of chlorine to kill both pathogens and good bacteria before returning to the environment. The heavy sludge that settles to the bottom of wastewater pools receives extra digestion in closed tanks filled with anaerobic bacteria.
Wastewater treatment anaerobes break down tough substances
like plant fibers and paper, but like rumen bacteria they emit methane gas. For many years, wastewater treatment plants burned off the methane stream from sludge digester tanks. Most now capture the gas and burn it for energy.
Methane production is a double-edged sword, a free energy
source and a greenhouse gas. When too much methane enters the atmosphere, it can be viewed as a type of waste like carbon monoxide
and hydrogen sulfides. A group of bacteria called methanotrophs use
methane for both carbon and energy and thus remove some of the
world’s excess methane. Methylobacteria, Methylococcus, and Xanthobacter live in many of the same places as methanogens and absorb the gas as it is produced. For example, still, swampy water contains small bubbles of methane from the methanogens living in the anaerobic sediments at the bottom. Methanotrophs live just above this region, capturing some of the methane as it rises. Xanthobacter possesses an additional ability to combine oxygen with hydrogen gas, also made by methanogens, in an explosive reaction called the knallgas reaction (O + 2 H → H O). Knallgas bacteria have sys—2
2
2
tems that control this reaction to make energy for the cell without blowing up!
Methanotrophs use the enzyme methane monooxygenase in its
metabolism. This enzyme also breaks down a toxic chlorinated solvent called trichloroethylene (TCE). TCE is a pollutant in soil and groundwaters and harms almost every system in the body. Electro-plating, metal degreasing, semiconductor manufacture, steel and rubber manufacturing, pulp and paper operations, and dry cleaning use TCE. Methanotrophs may soon become a tool for cleaning TCE
chapter 7 · climate, bacteria, and a barrel of oil
159
out of contaminated aquifers and land. If microbiologists do put methanotrophs to work in labs, they will avoid Xanthobacter; knallgas bacteria require the explosive mixture of hydrogen and oxygen.
Thiobacillus ferrooxidans presents an equally important but more complex involvement with pollution. T. ferrooxidans thrives in very acidic conditions and gets energy from inorganic iron-and sulfur-containing compounds. All of these features occur in mine tailings, the fluids that flow from ore and coal mines. Mine tailings cause considerable damage to stream and river ecosystems. T. ferrooxidans reacts with iron pyrite to make tailings even more acidic and caustic to the environment.
Mining site remediation currently uses chemicals to absorb or neutralize the acid, but sulfate-reducing bacteria offer an option because they alter the acid-producing reactions of T. ferrooxidans. Sulfate-reducing bacteria begin with the prefix “Desulfo-”—such as, Desulfococcus, Desulfovibrio, and Desulfobacter.
Despite T. ferrooxidans’s penchant for making a bad environmental situation worse, the species has been put to good use too. T. ferrooxidans recovers metal from ore deposits and also reduces sulfur in coal, a step in making low-sulfur or “clean coal.” Conventional coal burning releases the greenhouse gas sulfur dioxide from pyrite and
contributes to sulfuric acid formation in the atmosphere, the cause of
acid rain.
Depletion of high-grade metal ores in the United States has made
the recovery of low-grade ores critical to the metals industry. But high cost prevents the extraction of metals from low-grade ores by the usual smelting process. T. ferrooxidans and the similar species T. thiooxidans extract metals such as copper and uranium from ores filled with iron and sulfur compounds. For example, either of these species can recover copper from the copper-iron-sulfur compound
chalcopyrite. The bacteria do the recovery exclusively with enzymes,
an example of white biotechnology. Bacterial bioleaching also recovers some of the iron along with copper, and both are recycled by the metals industry into new products.
Similar mechanisms have been used to extract uranium from gold
ore. Bioleaching can recover 90 percent of the desired metal from low-grade ores and saves on the high energy cost of smelting.
160
allies and enemies
Bacteria on Mars
The ability of bacteria such as T. ferrooxidans to live surrounded by caustic chemicals or subsurface bacteria to eat rock keeps alive the notion that bacteria might live on other planets. Life on Mars represents more than an academic pursuit. Enzymes produced by potential Martian bacteria might have superior faculties to recover metals or
make energy from Earth’s greenhouse gases. Mars’ atmosphere
contains more than 95 percent carbon dioxide, about 3 percent nitrogen, and lesser amounts of oxygen, argon, and carbon monoxide.
Other than argon, bacteria on Earth use all of these gases. Earth’s autotrophs live on energy sources much like the elements on Mars, that is, silicon, iron, magnesium, calcium, sulfur, aluminum, potassium, sodium, and chlorine.
Recently microbiologists have developed a theory that the Earth’s
earliest bacteria may have degraded rock and thus carved out minia—
ture caves that may have served as protective habitats. Earth’s atmosphere 2.75 billion years ago, the estimated age of the caves, held no ozone layer to protect the breakdown of macromolecules by ultraviolet radiation. The caves would have shielded bacteria, protected their DNA from destruction, and provided a probable site for water condensation. The ancient anaerobes possibly used the minerals in the rock to which they attached and absorbed methane percolating
up from sediments. This scenario does not seem implausible knowing
what we do about extremophiles.
Birger Rasmussen of John Curtin University in Australia has
begun a global discussion on whether cave-dwelling bacteria indicate
similar bacteria might live on Mars. By analyzing the chemistry of ancient microbial deposits attached to the cave ceilings, he has presumed that the early bacteria used sulfur and methane, probably had access to water, and likely lived in a biofilm community.
Many scientists have been unwilling to make the giant leap from
bacteria on Earth to bacteria on other planets. Assuming extraterrestrial life follows the same biochemical principles as on Earth, bacteria on Mars would probably exist in the subsurface for the same reasons cave dwellers developed.
Theories abound on the possibilities of life on other planets in distant solar systems or on Mars. The three main themes of interplanetary under study in astrobiology are water, methane, and minerals.
