Life on a Young Planet (10 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

Knowing these simple relationships, we can work out the relative timing of events evident in a road cut, quarry, or mountainside. For example, in the geological cross section cartooned in
figure 4.2
, the oldest identifiable event is the deposition of bed
A
, followed sequentially by the formation of beds
B
through
F
. Later, these beds were folded and, after that, intruded by granite
G
. Then, after erosion planed off these older units, beds
H
through
J
were laid down. The fault on the right-hand side of the figure cuts all older sedimentary units and, therefore, must have occurred late. The youngest event we can infer from the cross section is the erosion that sculpted the current land surface.
Extension of such a local history to the global scale requires a means of establishing the time relationships of rock units found in different regions, say, in the Rockies and the Appalachians, or in North America and Australia. For rocks deposited since the Cambrian Explosion, fossils provide our best guide to stratigraphic correlation. Indeed, the eras, periods, and finer subdivisions of the geologic timescale (page 2) reflect more than anything else the changing composition of life through time. Sedimentary and volcanic rocks may also preserve distinctive features of chemistry or magnetism that complement or, in some cases, substitute for correlations based on fossils.
Fossils can reveal that two rocks units are the same age, but they cannot tell us what that age is. For that, we need a natural chronometer capable of recording quantitatively the passage of time. Radioactive isotopes bound into rock-forming minerals provide geology’s clocks.
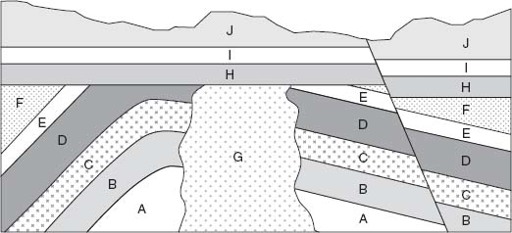
Figure 4.2.
A geological cross section, illustrating how geologists sort out relative age relationships. See text for discussion.
Radioisotopes are inherently unstable atoms that decay spontaneously to stable daughter elements at rates that can be measured accurately in the laboratory. This being the case, if we can determine how much of a radioactive parent element has disappeared from a mineral through time, or how much stable daughter has accumulated, we can calculate the age of the mineral itself. Interestingly, what remains constant in radioactive decay is the
proportion
of radioisotopes that decay in a set interval, not the number of atoms. Thus, as the abundance of a radioactive isotope in a mineral decreases through time, the absolute rate at which the isotope decays also declines. The pace at which a radioactive isotope decays is called its
half-life
—the time it takes for half of the radioisotope in a material to decay to another element.
Classically educated readers will be reminded of Xeno’s paradox, the ancient Greek puzzler in which Achilles chases a hare. Being a hero, Achilles runs faster than his prey, halving the distance between hunter and hunted each minute. When will Achilles catch the hare? The answer of course is never, because if the hare is moving at a constant speed, Achilles must continually be slowing down. Starting at a 200-yard disadvantage, he may race 200 yards in the first minute to the hare’s 100, but in minute two he’ll cover only 150 yards, and by minute four, he’ll make a mere 112.5 yards. Note that if we know how far Achilles has run
and how his speed varies with distance from the hare, we can figure out how long he’s been pursuing his frustrating chase. This, in essence, is how radiometric dating works.
The best-known radiometric dating system is provided by
14
C, or carbon 14, a rare isotope of carbon produced naturally by cosmic rays and anthropogenically by nuclear bombs. It decays to nitrogen (
14
N) with a half-life of 5,730 years. Because
14
C is so uncommon (it makes up fewer than one in every thousand carbon atoms) and its half-life so short, radiocarbon dating is limited to the past 100,000 years or so. In older materials, there simply isn’t enough
14
C left to measure accurately. In consequence,
14
C provides a great tool for Egyptologists or paleontologists interested in woolly mammoths, but it cannot help unravel Earth’s deep history.
To date the Warrawoona succession, we need a more stately clock—a radioisotope whose half-life is measured in many millions or even billions of years. Potassium 40 (
40
K) was recognized early on as a promising candidate for geochronology. This unstable isotope breaks down to form either calcium (
40
Ca), which unfortunately can’t be differentiated from calcium ions already in the mineral, or argon (
40
Ar), which can. The half-life of
40
K is 1.25
billion
years. Further, potassium is abundant and widely distributed in rock-forming minerals—it occurs in the feldspars that tint granites pink, in the microscopic minerals of volcanic ash, and in clays that form during weathering.
Despite these advantages, the potassium-argon chronometer is not much used by geologists interested in the early Earth. If
40
K behaves like a clock, tectonic and metamorphic processes act like toddlers eager to play with the dial. Geologic events that take place long after mineral formation can drive argon from minerals, resetting the clock and destroying the chemical memory of elapsed time. (An inert gas, argon is held only loosely within the chemical lattices of minerals.)
What we really need to date old rocks is a system that acts like the “black boxes” in airplanes—an isotope not easily lost from a mineral not readily altered. Zircons—uranium-bearing minerals found in granites and related volcanic rocks—are the flight recorders of Precambrian geology. In fact, the uranium bound into zircon crystals at their time of formation provides
two
reliable chronometers:
238
U decays to lead 206 (
206
Pb) with a half-life of about 4.5 billion years (the age of the Earth),
while the rarer isotope
235
U breaks down to
207
Pb with a half-life a bit longer than 700 million years. This provides a valuable cross-check of measured ages—if the two clocks don’t give the same age, the zircon has been altered.
If zircons have a problem, it is that they are
too
tough. Unlike most other minerals, zircons can go through the entire rock cycle, from crystallization in an igneous rock to metamorphism and subsequent erosion to form a sediment grain, without loss of chemical integrity. Indeed, magma ascending through the Earth’s crust can pluck zircons from surrounding rocks, incorporating older minerals (and, therefore, clocks) into younger rocks. What’s more, zircons can grow during each passage through the Earth’s interior; Archean
1
zircons may display a half dozen layers around a central core, each the accreted product of a specific geological event.
William Compston of the Australian National University developed an ingenious instrument for plumbing the radiometric complexities of ancient zircons. Called the Sensitive High Resolution Ion Microprobe (SHRIMP, for short—years ago, a fire in Compston’s lab prompted the predictable flurry of “throw a little SHRIMP on the barbie” jokes), this instrument uses fine ion beams to sample the individual growth layers of zircons so that geochemists can date each one independently. The SHRIMP revolutionized Archean geology, allowing geologists to unravel the complex time relationships of sedimentary, volcanic, and tectonic events on the early Earth.
Now we can understand why the basal Cambrian rocks along the Kotuikan River are said to be about 543 million years old. Locally, sedimentary rocks bearing the earliest Cambrian fossils are interbedded with a volcanic unit that contains zircons; U-Pb chronology shows that the zircons crystallized 543 ± 1 million years ago. (The “ ± 1” is an estimate of the error associated with the measured age; it is a statistical statement which indicates that the true age of crystallization has a 95 percent chance of falling within the interval 542–544 million years. Good
geologists pay careful attention to those error bars.) There are no well-dated rocks in the Spitsbergen succession, but fossils and chemical features of the Akademikerbreen Group permit at least broad correlation with better-dated rocks found elsewhere.
All of which brings us back to the age of the Warrawoona Group. SHRIMP analyses of zircons in volcanic rocks near the top and base of the group yield ages of 3,458 ± 2 and 3,471 ± 5 million years, respectively. Knowing these ages so precisely is a triumph of Earth science that imparts special importance to studies of Warrawoona life and environments.
In the late Precambrian rocks of Spitsbergen, biological signatures can be found almost everywhere—in microfossils, in stromatolites that catch the eye wherever we look, in biomarker molecules preserved in sedimentary organic matter, and in the isotopic abundances of carbon and sulfur in rocks throughout the succession. What does the paleobiological exploration of Warrawoona rocks reveal?
The sedimentary/volcanic succession at North Pole is, in fact, mostly volcanic and only a little sedimentary, not a good omen for the paleontologist. More than 95 percent of the unit consists of lavas poured out on land or in shallow water, along with ash layers and coarser beds made of fragmented volcanic rocks. Three and a half billion years ago, Warrawoona might have looked something like the volcanic necklace of the Indonesian Archipelago. In detail, however, Archean geography resists characterization by simple analogy to modern features.
Sediments, mostly preserved as dark cherts, accumulated in coastal basins nestled among the volcanoes. By now we know that chert lifts the paleontological heart, but the silica-rich rocks of North Pole formed in a manner quite different from those in Spitsbergen. These older cherts precipitated from volcanically heated fluids that percolated through Warrawoona sediments, replacing original minerals soon after deposition. Unfortunately for the paleontological heart (and mind), chert formed this way is as likely to destroy biological signatures as preserve them. Further complicating the issue, at least some of the cherts occur as veins that fill cracks in the sedimentary and volcanic pile. They appear to be part of a hydrothermal plumbing system, much like the network of underground conduits that feed present-day hot springs in Yellowstone Park.
Unraveling the depositional history of these rocks requires that one carefully map the distributions of constituent rock types and then use outcrop and laboratory observations to peer through the silica veil. Roger Buick did just that as a doctoral student at the University of Western Australia. Along with his Australian colleagues, Roger demonstrated that most Warrawoona sediments originated as muds, sands, and cobbles eroded from surrounding volcanoes and deposited in a flanking basin. From time to time, sandbars or other barriers blocked the basin’s mouth, restricting the flow of water into coastal lagoons. Evaporation increased the concentration of dissolved calcium and carbonate ions in these waters, leading to the production of calcium carbonate as whitings—millions of tiny crystals that formed in the water column, turning the lagoon milky white before they settled onto the seafloor as lime mud. Rosettes of gypsum crystals decorate many beds, deposited as evaporation proceeded still further. Actually, the gypsum in these rocks is long gone; its former presence is recorded by silica ghosts that preserve gypsum’s distinctive crystal form (
figure 4.3a
).
Perhaps the most unusual rocks in the North Pole region are beds of barium sulfate, or barite—fans and more continuous layers of slender prisms that grew like so much rock candy on the seafloor. Barite is uncommon in younger sedimentary successions, but Wouter Nijman and his colleagues at Utrecht University in the Netherlands believe that the large mounds of barite in the Warrawoona Group formed where hot hydrothermal fluids erupted onto the seafloor. From a human perspective, these eruptions seem alien and inhospitable, but to the heat-loving microorganisms found on early branches of the Tree of Life, they would have furnished a hot microbial Eden.
In the field, we can see wavy laminations in sedimentary rocks, much like the microbially laminated carbonates observed in Spitsbergen. In a few places, these laminations are bowed upward to form domes or cones—the geometric hallmarks of stromatolites (
figure 4.3b
). Warrawoona stromatolites were first reported in 1980 by Stanford University’s Don Lowe and, independently, by Australia’s Malcolm Walter, John Dunlop, and, of course, Roger Buick. The analogy with present-day stromatolite growth, noted in the preceding chapter, was pushed to the limit: in some of the oldest sedimentary rocks on Earth, these geologists discerned a familiar signature of biology.
