Life's Ratchet: How Molecular Machines Extract Order from Chaos (20 page)
Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann

The same can happen in our molecular world. If two large molecules stick to each other, their exclusion zones merge, and more space is available for the small guys. More space means more available microstates and thus increased entropy. Thus, the increase in
order
due to the binding of the large molecules is more than paid for by the increase in
disorder
arising from giving the surrounding smaller molecules more space to move, increasing the small molecules’ entropy. What we end up with is a force that is not the result of decreasing energy, but of increasing entropy. Such strange forces, which can only exist at the molecular scale, are called entropic forces.
Cells take advantage of these entropic forces to assemble a variety of molecular structures, including collagen, actin, and microtubule filaments. Collagen is the main ingredient of the extracellular matrix, the web of material outside our cells that holds our bodies together. Actin and microtubules are fibers that form the malleable skeleton of our cells.
How do we know biological fibers such as collagen are assembled by entropic forces? Remember the stock answer to any question of why something happens: because it minimizes free energy. Free energy, we have learned, is energy minus unusable energy. Unusable energy is given by the product of temperature and entropy. Since temperature comes into
the picture, we can do a relatively simple test to see if an assembly is driven by a reduction of energy or by an increase in entropy. If the assembly proceeds faster when we increase the temperature, it is mostly driven by a reduction in energy. On the other hand, if the assembly is
slowe
r when we increase the temperature, entropy is the culprit.
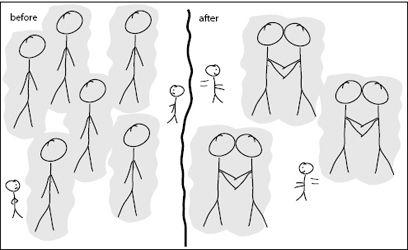
FIGURE 4.2.
If large molecules are separate from each other, their exclusion zones make it difficult for small molecules to move around. Once the larger molecules bind to each other, however, their exclusion zones overlap, creating more space for small molecules. This increases the entropy of small molecules and leads to an entropic force’s driving large molecules to bind to each other.
Most chemical reactions proceed faster at higher temperatures. Try to dissolve sugar or salt in ice water, and then try dissolving these substances in hot water, and you’ll see what I mean. Sugar dissolves much faster in hot water than in cold, indicating that the dissolution of sugar is driven by a reduction in energy. In the self-assembly of collagen, however, the reaction
slows down
when the temperature is increased. During collagen formation, total energy (
E
) actually
increases
when single collagen units combine to form fibers. Processes in which the energy of the system is increased should be unfavorable. But at the same time, the entropy (
S
) also increases. This increase in entropy more than trumps the unfavorable
energy increase, leading to an overall reduction in
free
energy (
F
=
E
−
TS
), because the entropy term (
TS
) is subtracted from the energy.
There are numerous biological examples of such entropy-driven self-assembly processes. In addition to collagen, actin, and microtubules, the self-assembly of viruses and the flagella of bacteria are entropy driven. These examples show that order can be created while increasing entropy. Entropy, as we have mentioned, is often confusingly equated with disorder. Well, it
is
a kind of disorder, but what we overlook in such a simplified picture is that the creation of disorder in one part of the system can be coupled to the creation of order in another part of the system. Just ask a snowflake.
Soap, detergents, and lipids are all examples of linear molecules that have one hydrophilic and one hydrophobic end. But what makes molecules hydro phobic or hydrophilic in the first place?
We know that oil and water don’t mix (unless you add eggs). Why? Water is a peculiar liquid, quite unlike any other liquid in the universe. For example, compared with liquids of similar molecular construction, water has to be heated to a much higher temperature to melt or boil. It also has the curious property that its solid form (ice) is lighter than its liquid form; this is why ice cubes float on your drink, rather than sink. For most other substances, the opposite is true: The liquid is lighter than the solid. All these strange properties of our favorite liquid are mainly due to hydrogen bonds. Hydrogen atoms of water molecules form strong bonds with oxygen atoms of neighboring water molecules, forming “bridges” between the molecules. Because these hydrogen bonds in liquid water are stronger than we would expect, water does not evaporate as easily as other liquids.
Hydrogen bonds arise because in each water molecule, which is made up of one oxygen atom and two hydrogen atoms, the oxygen “steals” the hydrogen’s electrons. The leftover hydrogen ions are positively charged, while the oxygen atom, now having two excess electrons, is negatively charged. When two neighboring water molecules come close to each other, the positively charged hydrogen on one molecule is attracted to the negatively charged oxygen on a neighboring water molecule. In liquid
water, these hydrogen bonds form and break continuously at a high rate. They are but fleeting couplings. Yet, they make a profound difference for the stability of the liquid and lead to its high boiling temperature. In ice, the hydrogen bonds are essentially permanent. They are also
directional
, that is, water molecules can only form hydrogen bonds in ice if the molecules are arranged in a certain way. This arrangement leaves large, molecule-sized voids in the ice structure, making ice less dense than liquid water. Hence, ice floats on water.
Nonwater molecules can also form hydrogen bonds with water, as long as they have some negatively charged parts. This includes many acids, sugars, and alcohols (a good thing—it would be a shame if alcohol and water didn’t mix). Other molecules, while not forming hydrogen bonds, are still quite happy in water, because they can accommodate their charges by surrounding themselves with water molecules. This is what ions do, and it explains why salt dissolves in water. Oils do not have any charges; they are a neutral bunch of molecules. Thus water cannot form hydrogen bonds with oil; nor are there any charges to accommodate. Oil is a molecular party-pooper—not very sociable. If oil is placed into water, water molecules form a kind of cage around an intruding oil molecule, trying to maximize hydrogen bonding to their own kind. The cage structure they form induces order in the water and therefore decreases entropy. A decrease in entropy leads to an increase in free energy, which is a bad thing. Trying to put oil into water comes at high (entropic) cost. What’s an oil molecule to do? Get out of the water and find its own kind. Soon enough, oil molecules form droplets, and eventually the droplets coalesce and leave the water all together. Oil and water separate.
The hydrophobic force, which is responsible for the separation of oil and water, is therefore another example of an entropic force. While the hydrophobic force separates oil from water, it also provides a powerful drive for oil molecules to come together and coalesce. In water, hydrophobic molecules attract each other. This attraction of hydrophobic molecules is an important mechanism for self-assembly. One tasty example of this is cheese. Cheese can be made by adding salt or acid to milk or, in a more modern approach, by adding rennin. Each method leads to essentially the same result, but interestingly, the physics of how each works is different. About 80 percent of the protein in cow’s milk is in the form of casein, a
phosphorus-containing protein. Casein molecules are hydrophobic, but also negatively charged. Their hydrophobic nature means that if they could get close enough to each other, the hydrophobic force would make them stick to each other. However, their negative charge keeps them apart. Now comes the cheese maker. Adding salt provides free positively and negatively charged ions to the milk. The positively charged ions crowd around each negatively charged casein molecule and shield its charge. Now casein molecules can approach each other and start to stick, forming cheese.
How about acid? Acid increases the amount of hydrogen ions in the milk, changing the milk’s pH. The positively charged hydrogen ions stick to the casein and neutralize it. Again the casein molecules start to stick and cheese is made.
Adding salt or acid, of course, affects the taste of the cheese, making it taste salty or sour. Sometimes this is not desirable. This is where rennin comes in. Rennin is an enzyme, a protein that promotes a certain reaction that cuts off the charged part of the casein. Without its charged part, the casein molecules are quickly forced together by the hydrophobic force.
The importance of hydrophobic forces is not limited to the kitchen. Hydrophobic forces play a crucial role in shaping the very molecules that keep us alive, as we will discuss in the following section.
A life-or-death example of a molecular self-assembly process is the folding of large biological molecules, especially proteins, into their active shapes. Proteins are made of long chains of chemical units called amino acids, strung together like pearls in a necklace (
Figure 4.3
). On Earth, every protein in every living organism uses just twenty different amino acids (there are many more amino acids that could in principle be used, but nature has decided to use only these twenty). The particular sequence of amino acids in each protein is encoded in the cell’s DNA. Different amino acids have different affinities, courtesy of the hydrophobic force. These tendencies to attract or repel determine the particular, coil-like shape of a protein. Get the folding of a protein wrong, and you could end up with Alzheimer’s disease, mad cow disease, or cystic fibrosis—examples of diseases in which
certain proteins fold into nonfunctional or even dangerous shapes, as a result of mutations or other unknown reasons.
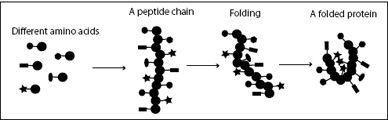
FIGURE 4.3.
Amino acids are molecules with identical backbones, but different side chains. The backbones can link together by peptide bonds, forming a peptide chain. This chain is pushed around by the molecular storm until it folds into a stable structure with hydrophobic side chains in the center (stars) and hydrophilic ones on the outside.
How does a protein find its optimal shape? When a protein is made by the molecular machinery of the cell, it initially comes out as a long string of amino acid beads. Some of these beads are destined to bind to each other, but initially the amino acid string wiggles around aimlessly, pushed by the molecular storm of bombarding water molecules (
Figure 4.3
). A molecule does not “know” how to fold—it needs to find its shape through random changes in its shape.
Even a small protein can fold into innumerable possible shapes, the number of which far exceeds the number of atoms in our universe. The energy of the protein depends on all the twists and turns of its chain and the contacts between amino acids along the chain. If we were to measure the twists of the chain (by assigning angles to each link of the chain), we could calculate the energy of the protein for any possible shape. Some shapes would have larger energies, some lower. The greatest number of amino acids bind together in the lowest-energy shapes. As shown in
Figure 4.3
, in the lowest-energy configuration, the hydrophobic amino acids are safely tucked inside, while the hydrophilic amino acids are found on the outside of the coil.
