Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (14 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

BOOK: Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine
3.6Mb size Format: txt, pdf, ePub
•
Early diastolic decrescendo murmur at LUSB
(RUSB if dilated Ao root); ↑ w/ sitting forward, expir, handgrip; severity of AI ∝ duration of murmur (except in
acute and severe late);
Austin Flint murmur
: mid-to-late diastolic
rumble at apex (AI jet interfering w/ mitral inflow)
•
Wide pulse pressure
due to ↑ stroke volume, hyper-dynamic pulse → many of classic signs (see table); pulse pressure narrows in late AI with ↓ LV fxn; bisferiens (twice-beating) arterial pulse • PMI diffuse and laterally displaced; soft S
1
(early closure of MV); ± S
3
(≠ ↓ EF but rather just volume overload in AI)
Diagnostic studies
• ECG: can see LVH, LAD, abnl repol; CXR: cardiomegaly ± ascending Ao dilatation •
Echo
: severity of AI (severe = width of regurgitant jet >65% LVOT, vena contracta >0.6 cm, regurg fraction ≥50%, regurg orifice ≥0.3 cm
2
, flow reversal in descending Ao); LV size & fxn
Treatment (
Circ
2008;118:e523;
EHJ
2012;33:2451)
• Acute decompensation (consider ischemia and endocarditis as possible precipitants):
surgery
usually urgently needed for acute severe AI which is poorly tolerated by LV
IV afterload reduction (nitroprusside) and inotropic support (dobutamine)
± chronotropic support (↑ HR → ↓ diastole → ↓ time for regurgitation)
pure vasoconstrictors and IABP contraindicated
• In chronic AI, management decisions based on
LV size and fxn
(and before sx occur) •
Surgery (AVR
, replacement or repair if possible)
sx
(if equivocal, consider stress test)
severe AI
(if
not
severe, unlikely to be cause of sx)
asx severe AI
and
EF
≤
50%
or
LV dilation
(end syst. diam. >50–55 mm or end diast. diam. >70–75 mm, esp. if progression) or undergoing cardiac surgery
• Transcatheter AoV replacement (TAVR) being explored (
JACC
2013;61:1577) • Medical therapy:
vasodilators
(nifedipine, ACEI/ARB, hydralazine) if severe AI w/ sx or LV dysfxn & Pt not operative candidate or to improve hemodynamics before AVR; no clear benefit on clinical outcomes or LV fxn when used to try to prolong compensation in asx severe AI w/ mild LV dilation & nl LV fxn (
NEJM
2005;353:1342)
MITRAL REGURGITATION (MR)
Etiology (
Lancet
2009;373:1382;
NEJM
2010;363:156)
•
Leaflet abnormalities
:
myxomatous degeneration (MVP)
, endocarditis, calcific
RHD, valvulitis (collagen-vascular disease), congenital, anorectic drugs, XRT
•
Functional
: inferoapical
papillary muscle displacement due to ischemic LV remodeling
or other causes of DCMP; LV
annular dilation
due to LV dilation • Ruptured chordae tendinae: myxomatous, endocarditis, spontaneous, trauma • Acute papillary muscle
dysfxn
b/c of ischemia or
rupture
during MI [usu. posteromedial papillary m. (supplied by PDA only) vs. anterolateral (suppl. by diags & OMs)]
• HCMP: (see “Cardiomyopathy”)
Clinical manifestations
• Acute:
pulmonary edema
, hypotension, cardiogenic shock (
NEJM
2004;351:1627) • Chronic: typically asx for yrs, then as LV fails → progressive DOE, fatigue, AF, PHT
• Prognosis: 5-y survival w/ medical therapy is 80% if asx, but only 45% if sx
Physical exam
•
High-pitched
,
blowing
,
holosystolic murmur at apex
; radiates to axilla; ± thrill; ↑ w/ handgrip (Se 68%, Sp 92%),
↓ w/ Valsalva (Se 93%) (
NEJM
1988;318:1572)
ant. leaflet abnl → post. jet heard at spine
post. leaflet abnl → ant. jet heard at sternum
• ± diastolic rumble b/c ↑ flow across valve • Lat. displ. hyperdynamic PMI, obscured S
1
, widely split S
2
(A
2
early b/c ↓ LV afterload, P
2
late if PHT); ± S
3
• Carotid upstroke brisk (vs. diminished and delayed in AS)
Diagnostic studies (
NEJM
2005;352:875)
• ECG: may see LAE, LVH, ± atrial fibrillation • CXR: dilated LA, dilated LV, ± pulmonary congestion •
Echo
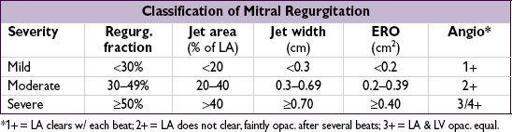
: MV anatomy (ie, etiol); MR severity: jet area (can underestimate eccentric jets), jet width at origin (vena contracta) or effective regurgitant orifice (ERO; predicts survival); LV fxn (EF should be
supranormal
if compensated, ∴ EF <60% w/ sev. MR = LV dysfxn); TEE if TTE inconclusive or pre/intraop to guide repair vs. replace •
Cardiac cath
: prominent PCWP
c-v
waves (not spec. for MR), LVgram for MR severity & EF
Treatment (
Circ
2008;118:e523;
NEJM
2009;361:2261;
EHJ
2012;33:2451)
• Acute decompensation (consider ischemia and endocarditis as precipitants)
IV afterload reduction (nitroprusside), ± inotropes (dobuta), IABP, avoid vasoconstrictors
surgery
usually needed for acute severe MR as prognosis is poor w/o MVR
•
Surgery
(repair [preferred if feasible] vs. replacement w/ preservation of mitral apparatus)
sx severe MR
,
asx severe MR
and
EF 30–60%
or
LV sys. diam.
>
40 mm
consider MV
repair
for asx severe MR w/ preserved EF, esp. if new AF or PHT
if AF, maze procedure or pulm vein isolation may → NSR and prevent future stroke
• In Pts undergoing CABG w/ mod–sev fxnal MR, consider annuloplasty ring • Percutaneous MV repair: edge-to-edge clip less effective than surgery, but ? consider for elderly, fxnal MR or low EF (
NEJM
2011;364:1395); percutaneous valve under study • Medical: clinical benefit in asx Pts; bB preserve LV fxn (
JACC
2012;60:833); if sx but not operative candidate ↓
preload
(↓ HF and MR by ↓ MV orifice): diuretics, nitrates (esp. if ischemic/fxnal MR); if LV dysfxn: ACEI, bB, ± BiV pacing; maintain SR
MITRAL STENOSIS (MS)
Etiology (
Lancet
2012;379:953)
•
Rheumatic heart disease
(RHD):
fusion of commissures
→ “fish mouth” valve
from autoimmune rxn to b strep infxn; seen largely in developing world today
•
Mitral annular calcification
(MAC): encroachment upon leaflets → functional MS
• Congenital, infectious endocarditis w/ large lesion, myxoma near MV, thrombus • Valvulitis (eg, SLE, amyloid, carcinoid) or infiltration (eg, mucopolysaccharidoses)
Clinical manifestations (
Lancet
2009;374:1271)
•
Dyspnea and pulmonary edema
(if due to RHD, sx usually begin in 30s)
precipitants: exercise, fever, anemia, volume overload (incl. pregnancy), tachycardia, AF
•
Atrial fibrillation
: onset often precipitates heart failure in Pts w/ MS
•
Embolic events
: commonly cerebral, esp. in AF or endocarditis • Pulmonary: hemoptysis, frequent bronchitis (due to congestion), PHT, RV failure • Ortner’s syndrome: hoarseness from LA compression of recurrent laryngeal nerve
Physical exam
•
Low-pitched mid-diastolic rumble at apex
w/ presystolic accentuation (if not in AF); best heard in L lat decubitus position during expi-ration, ↑ w/ exercise; severity proportional to
duration
(not intensity) of murmur •
Opening snap
(high-pitched early diastolic sound at apex) from fused leaflet tips;
Other books
Officer Down (A Digital Short Story) by DeLee, David
Kindergarten by Peter Rushforth
Hostage of the Hawk by Sandra Marton
That's Amore by McCarthy, Erin
New and…Improved? & Andrew in Excess by Jill Shalvis
The Fiend by Margaret Millar
After Days (The After Days Trilogy) by Medbury, Scott
Beanball by Gene Fehler
Steel and Lace (Lace Series) by Leigh, Adriane
A Lover's Mask by Altonya Washington