Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (74 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

• Primary effusion lymphoma (<5%): HHV8 driven; also can be seen in other immuno-supp. Pts such as s/p solid organ transplant or w/ chronic HBV. Treat with standard CHOP (often CD20–), but poor prognosis.
PLASMA CELL DYSCRASIAS
MULTIPLE MYELOMA (MM)
Definition and epidemiology
(
NEJM
2011;364:1046)
• Malignant neoplasm of
plasma cells
producing a monoclonal Ig = “
M protein
”
•
21,700 new cases and
10,710 deaths/y in U.S. (2012); median age at diagnosis 69 y • African American:Caucasian ratio2:1
Clinical manifestations
(CRAB criteria)
• Hyper
C
alcemia due to ↑ osteoclast activity •
R
enal disease: multiple mechanisms include toxic effect of filtered light chains →
renal failure
(cast nephropathy) or
type II RTA
; amyloidosis or light chain deposition disease →
nephrotic syndrome
; hypercalcemia, urate nephropathy, type I cryoglobulinemia •
A
nemia (normocytic) due to bone marrow involvement and autoimmune Ab •
B
one pain due to ↑ osteoclast activity → lytic lesions, pathologic fx • Recurrent infxns due to relative hypogammaglob. (clonal plasma cells suppress nl Ig) • Neurologic: cord compression; POEMS (
p
olyneuropathy,
o
rganomegaly,
e
ndocrinopathy,
M
protein,
s
kin changes) syndrome • Hyperviscosity: usually when IgM >4 g/dL, IgG >5 g/dL, or IgA >7 g/dL
• Coagulopathy: inhibition of or Ab against clotting factor; Ab-coated platelets • Amyloidosis (see “Amyloidosis”)
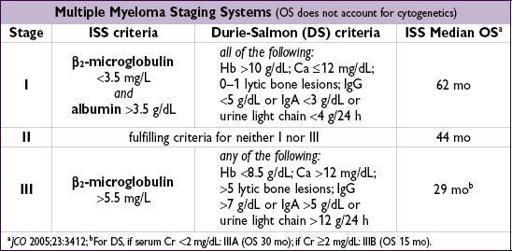
Diagnostic and staging evaluation
•
Symptomatic MM criteria
(all 3 must be met)
1) M protein in serum or urine (no specific level required)
2) bone marrow clonal plasmacytosis (≥10%) or presence of a plasmacytoma
3) myeloma-related organ or tissue impairment (ROTI) = lytic bone lesions, Ca >11.5 g/dL, Cr >2 mg/dL, or Hb <10
•
Variants
smoldering MM: M protein >3 g/dL and/or plasmacytosis >10%, but asx & no ROTI risk of prog.: M protein concen., subtype & free light chain ratio (
NEJM
2007;356:2582)
solitary bone plasmacytoma: 1 lytic lesion w/o M protein, plasmacytosis, or other ROTI
extramedullary (nonosseous) plasmacytoma: usually upper respiratory tract
plasma cell leukemia: plasma cell count >2000/µL in peripheral blood
nonsecretory MM (~2% of MM Pts): no M protein, but marrow plasmacytosis & ROTI
• Ddx of M component: MM, MGUS (see below), CLL, lymphoma, cirrhosis, sarcoidosis, RA • Peripheral smear → rouleaux (see insert); ✓ Ca, alb, Cr; ↓ anion gap, ↑ globulin, ↑ ESR
•
Protein electrophoresis and immunofixation
serum protein electrophoresis (SPEP)
: quantitates M component;in ~80% of Pts
urine protein electrophoresis (UPEP): detects the ~20% of Pts who secrete only light chains ( = Bence Jones proteins), which are filtered rapidly from the blood
immunofixation: shows component is monoclonal and identifies Ig type → IgG (50%), IgA (20%), IgD (2%), IgM (0.5%), light chain only (20%), nonsecretors (<5%)
serum-free light chain assay
: important test for dx and follow-up of response to Rx
• β
2
-microglobulin and LDH levels reflect tumor burden •
BM bx cytogenetics
: normal karyotype better than abnl.
Standard risk
= hyperdiploidy or t(11;14);
high risk
= hypodiploidy, del. 17p13 (~10% of Pts), t(4;14) & t(4;16) • Gene mutations include
TP53
,
NRAS
,
KRAS
,
BRAF
& NK-kB pathway (
Nature
2011;471:467) •
Skeletal survey
(plain radiographs) to identify lytic bone lesions and areas at risk for pathologic fracture;
bone scan is not useful for detecting lytic lesions
Treatment
(
NEJM
2011;364:1046;
Am J Hematol
2012;87:79)
• Not indicated for smoldering MM or asx stage I disease • Decisions generally dictated by
risk stratification
and
transplant eligibility
• Active agents include: bortezomib (V), dexamethasone (D), prednisone (P), lenalidomide (R), thalidomide (T), melphalan (M), cyclophosphamide (C), doxorubicin, carfilzomib (Cz) • Induction Rx regimens w/ best response rate incl. those w/ proteasome inhib (V, Cz) & immunomod (R), but many 2-or 3-drug options used based on comorbidities and risk. Proteasome inhib containing regimens incl. MPV, RVD, VCD & CzRD.
• If
not
transplant eligible:
induction chemo
↑ survival, not curative; consider maint chemo • If transplant
eligible
: induction chemo (eg, RVD, VCD, RD, VTD;
Lancet
2010;376:2075) then
high-dose chemo
+
auto-HSCT
. Not curative, but ↑ survival c/w chemo (
NEJM
2009;360: 2645). Timing of HSCT (upfront vs. relapse) under study. Offer if <70 y w/ good perf. status & no prohibitive comorbidities. Maint Rx w/ R or V until progression or intolerance. Role of tandem auto-HSCT & allo-HSCT remains controversial (
NEJM
2003;349:2495).
• Local radiation for solitary or extramedullary plasmacytoma • Adjunctive Rx
bone
:
bisphosphonates
(
JCO
2007;25:2464); XRT for sx bony lesions
renal
: avoid NSAIDs & IV contrast; consider plasmapheresis for acute renal failure
hyperviscosity syndrome
: plasmapheresis;
infxns
: consider IVIg for recurrent infections
• Common
toxicities
of Rx: melphalan → myelosuppression; lenalidomide → low plts & thromboembolism; bortezomib → periph. neuropathy; steroids → hyperglycemia, infxn
MONOCLONAL GAMMOPATHY OF UNCERTAIN SIGNIFICANCE (MGUS)
Definition and epidemiology
(
NEJM
2006;355:2765)
• M protein <3 g/dL, no urinary Bence Jones proteins, marrow plasmacytosis <10%, no ROTI • Prevalence
3% in population >50 y of age,
5% in population >70 y of age, and
7.5% in population >85 y of age (
NEJM
2006;354:1362)
Management
• ✓ CBC, Ca, Cr, SPEP, serum free light chains, UPEP w/ immunofixation (to exclude MM) • Close observation: repeat SPEP in 6 mo, then yearly thereafter if stable
Prognosis
(
NEJM
2002:346:564)
•
1%/y or
25% lifetime risk → MM, WM, amyloidosis, or malign. lymphoproliferative dis.
• Abnormal serum-free light chain ratio: ↑ risk of progression to MM (
Blood
2005;105:812)
WALDENSTRÖM’S MACROGLOBULINEMIA (WM)
Definition
(
Blood
2009;114:2375)
• B-cell neoplasm (lymphoplasmacytic lymphoma) that secretes monoclonal IgM
•
MYD88
(NF-кB pathway) L265P somatic mutation found in 91% of Pts w/ WM and could be used to distinguish WM from MM (
NEJM
2012;367:826) •
No evidence of bone lesions
(IgM M component + lytic bone lesions = “IgM myeloma”)
Clinical manifestations
•
Fatigue
from anemia is most common sx •
Tumor infiltration
: BM (cytopenias), hepatomegaly, splenomegaly, lymphadenopathy •
Circulating monoclonal IgM
hyperviscosity syndrome
(~15%)
neurologic: blurred vision (“sausage” retinal veins on funduscopy), HA, dizziness, Δ MS
cardiopulmonary: congestive heart failure, pulmonary infiltrates
type I
cryoglobulinemia
→
Raynaud’s phenomenon
platelet dysfxn → mucosal bleeding
•
IgM deposition
(skin, intestine, kidney); amyloidosis and glomerulopathy •
Autoantibody activity of IgM
chronic AIHA (prominent
rouleaux
; 10% Coombs’= AIHA)
peripheral neuropathy
: may be due to IgM against myelin-associated glycoprotein
Diagnostic evaluation
• SPEP + immunofixation with IgM >3 g/dL; 24-h urine for UPEP (only 20% haveUPEP) • Bone marrow biopsy: ↑ plasmacytoid lymphocytes; β
2
-microglobulin for prognostic eval •
Relative serum viscosity
: defined as ratio of viscosity of serum to H
2
O (nl ratio 1.8) hyperviscosity syndrome when relative serum viscosity >5–6