Resident Readiness General Surgery (43 page)
Read Resident Readiness General Surgery Online
Authors: Debra Klamen,Brian George,Alden Harken,Debra Darosa
Tags: #Medical, #Surgery, #General, #Test Preparation & Review

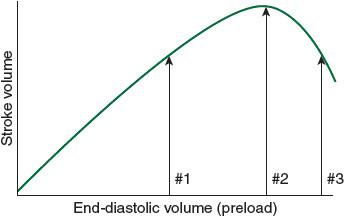
Figure 34-2.
The Frank-Starling relationship, illustrating how increased preload results in increased stroke volume (and, hence, cardiac output).
The Frank-Starling relationship (see
Figure 34-2
): As preload increases, the myocardium stretches and cardiac output subsequently increases to a point where the myocardium becomes overstretched and stroke volume and cardiac output begin to decline. The #1 arrow represents the point on the curve where additional intravascular volume (preload) will augment cardiac output. The #2 arrow represents the equilibrium point, dependent on the specific patient’s ventricular compliance where preload optimizes cardiac output. The #3 arrow represents postoperative fluid overload where too much preload overstretches the myocardium and actually decreases cardiac output resulting in heart failure.
Afterload
: Afterload is perhaps best thought of as the pressure the heart has to pump blood out against. In most cases, it is directly related to the blood pressure and systemic vascular resistance. A common scenario is the patient with mild-to-moderate compensated heart failure and longstanding hypertension who develops a hypertensive crisis postoperatively, possibly secondary to pain, and discontinuation of home antihypertensives. The already weak heart is then forced to pump against a much higher afterload resulting in decreased forward flow and flash pulmonary edema.
Contractility/intrinsic pump function
: Once preload and afterload are optimized, attention should be turned to contractility. The most concerning cause of decreased contractility is myocardial ischemia or infarction. As the myocardium becomes ischemic, it loses its ability to contract and force blood forward. Any time postoperative heart failure is encountered, one must ensure that it is not the result of myocardial ischemia. Another factor resulting in decreased intrinsic pump dysfunction and inability to move blood forward may be underlying valvular heart disease. Although a discussion of the intricacies of managing different types of valvular heart disease is beyond the scope of this book, it is important to consider this in one’s workup, including the possibility of acute mitral regurgitation as a result of papillary muscle rupture in the setting of myocardial infarction.
Heart rate and rhythm
: As mentioned, the second component of cardiac output is heart rate. Patients who develop heart block postoperatively with bradycardic underlying escape rhythms can also develop heart failure. It is important to remember that myocardial ischemia should also always be considered with new-onset heart block. Tachyarrhythmias represent a unique entity and they are discussed here as part of “rhythm disturbances”; however, their effects are actually on preload. Based on the equation defined above (
Figure 34-1
), it would seem that tachyarrhythmias should result in increased cardiac output because of increased heart rate. In the case of supraventricular tachycardias (SVT), and atrial fibrillation with rapid ventricular response, however, the result is often a drop in cardiac output. The mechanism for this phenomenon is a decrease in diastolic filling time that subsequently decreases end-diastolic volume (preload) and, therefore, cardiac output. Atrial fibrillation has the added effect of removing the “atrial kick” that can contribute anywhere from 20% to 30% to cardiac output because it augments ventricular filling. This augmentation is particularly critical for patients with long-standing hypertension and subsequent thickened myocardium with decreased ventricular compliance. When these patients suddenly flip into atrial fibrillation in the postoperative period, the result may be decompensated heart failure.
In summary, when thinking about the etiology of postoperative heart failure and its most common underlying pathophysiology, the following should be considered, in this order:
A. Too much preload—fluid overload, likely iatrogenic in the setting of aggressive postoperative resuscitation
B. Too much afterload—uncontrolled hypertension in the postoperative period
C. Decreased cardiac output (myocardial dysfunction)—most commonly from myocardial ischemia/infarction
D. Rhythm disturbance tachyarrhythmias such as atrial fibrillation, or bradyarrhythmias from new or chronic heart block
2.
With regard to afterload, Mr. Jones has underlying hypertension suggesting that if his blood pressure is not adequately controlled in the postoperative period, he is at risk for a hypertensive crisis, subsequent heart failure, and flash pulmonary edema. With regard to contractility, the patient is a smoker and long-standing diabetic. These two factors alone significantly increase his risk of coronary artery disease making him vulnerable to myocardial ischemia in the postoperative period. Additionally, his long-standing history of poorly controlled hypertension not only increases his risk of coronary disease but also suggests that his left ventricle has been pumping against an increased afterload for many years. This typically results in some degree of left ventricular hypertrophy. As a result, he will likely have a stiff ventricle and some degree of diastolic cardiac dysfunction. Finally, with regard to rhythm, his increased left ventricular pressures will also likely have caused some degree of left atrial dilatation, placing him at increased risk for atrial fibrillation. Should he develop postoperative atrial fibrillation, he will be more likely to develop decompensated heart failure as he depends on his atrial kick to fill his left ventricle.
3.
Returning to the underlying physiology of the disease process, one must keep in mind that all the physical exam findings seen in heart failure will be the result of the heart’s inability to maintain forward flow of blood and perfusion to the body. As systemic perfusion decreases, one might expect to see cold and mottled extremities and perhaps decreased urine output. The cardiac exam may reveal an unusual rhythm or new murmur. Classically, an S3 gallop may also be heard. As left-sided failure results in pulmonary edema, the associated findings of basilar crackles and increased work of breathing will be seen. Hypoxemia is likely to result. When left-sided failure begins to result in right-sided failure, it is common to see distended neck veins, increasing peripheral edema, and perhaps RUQ pain and a hepatojugular reflex as the patient develops a congestive hepatopathy. If the patient is in an ICU and has a central venous or pulmonary artery catheter in place, one would expect to see elevated filling pressures (central venous pressure and wedge pressure) and a low cardiac output or index.
4.
Again, our original framework can be applied to laboratory testing. A brain natriuretic peptide may be helpful in confirming fluid overload (too much preload). An EKG will help not only to evaluate for myocardial ischemia but also to elucidate any underlying rhythm abnormality. Troponins can also be sent to evaluate for ischemia. Serum electrolyte evaluation and appropriate repletion is crucial in the setting of arrhythmias. Patients often manifest decompensated heart failure with respiratory distress, and in those cases, a thorough workup with an arterial blood gas and CXR is often warranted to look for noncardiac causes of hypoxemia. Finally, once the patient is stabilized, an echocardiogram may provide insight into underlying myocardial and valvular function.
5.
As always, the approach to stabilizing a patient should begin with the ABCs. Although these patients will typically not have airway issues, restoring ventilation and oxygenation should be a priority if there is a component of pulmonary edema. An arterial blood gas and CXR should immediately be obtained. As mentioned, an EKG will be useful in ruling out arrhythmias, conduction abnormalities, and ischemia as the cause of heart failure. If the patient is hemodynamically unstable, transfer to the ICU for inotropic support and invasive hemodynamic monitoring will likely be necessary and should be anticipated early. In the case of acute hemodynamic instability, the likely cause is an arrhythmia or ischemia. In the case of bradyarrhythmias, pacing pads should be placed and any nodal blockade should be reversed. Glucagon can be used to reverse the effects of β-blockers, and calcium can be used to reverse the effects of calcium channel blockers and improve myocardial contractility. Atropine or isoproterenol is also very effective for increasing heart rate. In the case of tachyarrhythmias with hemodynamic instability, the patient will need to be cardioverted and appropriate antiarrhythmics such as amiodarone and β-blockers started. If myocardial ischemia is suspected, an ACS protocol should immediately be instituted. In all of these cases, prompt evaluation by a cardiology service is beneficial. It is also important to note that a more senior resident should be notified of the patient’s clinical status. Ultimately, the approach to managing postoperative heart failure begins by stabilizing the patient, thinking through the pathophysiology (ie, preload, afterload, contractility, and rate/rhythm), and then treating accordingly.
TIPS TO REMEMBER
In most cases, postoperative heart failure is the result of one of the following four etiologies:
Too much preload—that is, fluid overload
Too much afterload—that is, uncontrolled hypertension
Poor contractility—likely related to ischemia resulting in decreased cardiac output
Other books
Cinderella Ate My Daughter by Orenstein, Peggy
We Won't Feel a Thing by J.C. Lillis
Muchacho by Louanne Johnson
Teach Me by R. A. Nelson
Harlequin Nocturne March 2016 Box Set by Megan Hart
Brenda Hiatt by Scandalous Virtue
The Accidental Marriage by Sally James
An Untamed State by Roxane Gay
Celtika by Robert Holdstock
The Master Butcher's Singing Club by Louise Erdrich
