The Case for Mars (33 page)

One way might be for the cart to use a microwave device to heat the soil below it. This would cause the water in the soil to vaporize and rise up as steam. The cart would carry a kind of canopy with a flexible skirt brushing the ground all around it. This skirt would act as a sufficiently good seal to hold the water vapor until most of it frosted out on the canopy roof, after which it could be collected for use. The advantage of this scheme is that no digging is required, and furthermore, microwaves can be tuned so that they put most of their energy into heating the water molecules, instead of wasting power by heating water and dirt indiscriminately. Unfortunately, the rising vapor will transfer heat to the soil, so that in the end much of the heat ends up wasted anyway (although not as much as in a purely thermal heating system). The problem, however, is that the microwave power input must be electrical, not thermal. The 6,000 W of waste heat the RTG produces can’t be used to drive the system, only the unit’s 300 W of electrical output. Thus, even if one watt of microwave power should prove twice as efficient as thermal power in driving water out of soil, you still come out with only one tenth the output because thermal power is twenty times more available. If the water concentration was very high, however, and the ground too strong to break (as would be the case with permafrost) this system might work better than a mobile di
gger, though its output would still be rather low. For example, let’s assume we operate such a system over a permafrost deposit that is 30 percent water by weight. We estimate that about 1 kWe-hr would be needed to extract each kilogram of water. So, over the course of a Martian sol (24.6 terrestrial hours), the microwave cart driven by a 300-watt RTG could extract about 7.4 kilograms of water. The only way to improve on this performance would be to apply a lot more power, perhaps by connecting the cart by a long cable to the base’s nuclear reactor and applying 100 kWe. In that case, 2,200 kilos of water per day could be produced, but mobility would be lost.
FIGURE 7.4
Mobile methods of extracting water from Martian soil: (a) soil eater on wheels; (b) mobile microwave system with shirt; (c) port greenhouse dome with condenser. (Artwork by Michael Carroll.)

I think a better solution would be to put a transparent tent over a selected area of Martian terrain and warm the inside via the greenhouse effe
ct that would occur naturally within. The greenhouse heating could be augmented by positioning large, lightweight reflectors around the tent, and moving them with the Sun to maximize the solar heating of the enclosed area. Inside the tent, the soil would be heated, not to 500°C certainly, but far above its average temperature. This would cause a fraction of the adsorbed water the soil contains to out-gas, and the moisture released could be captured as frost on a cold plate kept refrigerated in one corner of the tent (just like the frost build-up in your freezer). To see how effective such a system might be, consider that the average solar incidence on Mars is 500 watts per square meter (W/m
2
). If the tent is a hemisphere 25 meters in diameter, and the tent greenhouse plus reflector arrangement causes the equivalent of an extra 200 W/m
2
of heating to occur within the tent, the total effective power of the system would be 98 kW This is enough to release 224 kilograms of water from 3 percent-grade soil in the course of an eight-hour day. This amount of water would be available within the first half centimeter of soil within the tent. Made of 0.1 mm thick polyethylene, the tent would have a mass of only 100 kilograms (and therefore weigh 38 kilograms on Mars), so it could be carried about by rover crews to a new position every day. After the tent moved on, the mined surface soil would rehydrate itself naturally, allowing the same field to be repeatedly “farmed” for water.
A completely different approach would entail extracting water from the Martian atmosphere. The problem here is that the air on Mars is very dry—under typical conditions you have to process one million cubic meters of Martian air to acquire one kilogram of water. In a classic paper, engineer Tom Meyer and Mars scientist Chris McKay proposed a mechanical compressor system capable of doing just that.
26
The authors found that every kilogram of water produced would requ
ire abou
t 103 kWh of electrical energy. Comparing this result to the figures for the soil-based water-extraction systems described above (about 3.5 kWh of thermal energy per kilogram), it certainly seems unattractive, although it should be pointed out that the compressor system will also produce a lot of useful argon and nitrogen from the atmosphere for base life support. More recently, however, Adam Bruckner, Steven Coons, and John Williams of the University of Washington undertook a study in which instead of compressing the air, they simply employed a fan to blow
it against a zeolite sorption bed.
27
Zeolite is an extreme desiccant, and can be used to reduce atmospheric water vapor concentrations to a few parts per billion, well below even Martian humidity. At Martian temperatures, zeolite will adsorb up to 20 percent of its weight in water. Once the zeolite is saturated, you can bake the water out at an energy cost of about 2 kWh of thermal energy per kilogram, after which the now desiccated zeolite can be used again. Since all you have to do is move the air, not compress it, the mechanical fan power is much less than the pump power needed by the Meyer and McKay system, perhaps requiring another 2 kWh of electrical energy per kilogram of water processed. Energy costs are thus comparable to the systems based upon soil water extraction. The main problem with any atmosphere-based water-extraction system on Mars, however, is that it must be rather large to achieve a useful level of output. For example, a system pairing an intake ductth a 10 square meter cross-sectional area and a fan capable of generating an intake air speed of 100 meters per second (close to 200 miles per hour) would produce just 90 kilograms or so of water per day. However, since the machine does not need to be mobile, the 8 kWe needed to run the fan could readily be provided by the base power supply. This, taken together with the facts that no digging or prospecting is required, the system is susceptible to complete automation, and the raw material, air, is infinitely renewable, may ultimately make such atmospheric water extraction systems quite attractive.
All in all, while there may not be enough water available on Mars to support Lowell’s visions of water-bearing canals criss-crossing the planet, there is certainly enough available to support a Mars outpost. No doubt much of the water tapped from Mars’ arid environs will go to adding a touch of green to the Red Planet.
GREEN THUMBS FOR THE RED PLANET
Given the costs of interplanetary transportation, it is obvious that if significant human populations are to settle on other worlds, they will eventually have to grow their own food. In this respect, Mars stands at an enormous advantage to the Earth’s Moon and every other known extraterrestrial body. Of the four main elements comprising organic matter—hydrogen, carbon, nitrogen, and oxygen—all are readily avail
able on Mars. It’s been argued that asteroids are likely to contain carbonaceous material, and some evidence has been presented from the
Clementine
mission indicating that the Moon may harbor ice deposits in permanently shaded areas near its south pole. But these arguments miss the point, because the biggest problem with the Moon, as with all other airless planetary bodies and proposed artificial free-space colonies (such as those proposed by Gerard O’Neill
28
) is that
sunlight is not available in a form useful for growing crops
. This is an extremely impo
rtant point and it is not well understood. Plants require an enormous amount of energy which can only come from sunlight. For example, a single square kilometer of cropland on Earth is illuminated with about 1,000 MW of sunlight at noon, a power load equal to an American city of one million people. Put another way, the amount of power required to generate the sunlight responsible for the crop output of the tiny country of El Salvador exceeds the combined capacity of every power plant on Earth. Plants can stand a drop of perhaps a factor of five in their light intake compared to terrestrial norms and still grow, but the fact remains: The energetics of plant growth make it inconceivable to raise crops on any kind of meaningful scale with artificially generated light. That said, the problem with using the natural sunlight available on the Moon or in space is that it is unshielded by any atmosphere. (The Moon has an additional even more intractable problem with its twenty-eight-day light/dark cycle, which is completely un acceptable to plants.) Thus, plants grown in a thin-walled greenhouse on the surface of the Moon or an asteroid would be killed by solar flares. In order to grow plants safely in such an environment, the walls of the greenhouse would have to be made of glass 10 centimeters thick, a construction requirement that would make the development of significant agricultural areas prohibitively expensive. Using reflectors and other light-channeling devices would not solve this problem, as the reflector areas would have to be enormous, essentially equal in area to the crop domains, creating preposterous engineering problems if any significant acreage is to be illuminated.
Mars’ atmosphere, on the other hand, is sufficiently dense to protect crops grown on the surface against solar flares. On Mars, as we have seen, large inflatable greenhouses protected by geodesic domes could be readil
y deployed, rapidly creating huge temperate-environment domains for crop growth. Martian sunlight levels, at 43 percent those of Earth, are entirely adequate for photosynthesis, which in fact could be accelerated relative to Earth by filling the domes with higher concentrations of carbon dioxide than are available on Earth. We have seen that a 1-mm thick Kevlar reinforced dome fabric would be needed to support a 50-meter diameter habitation pressurized to 5 psi. However, plants require only 0.7 psi, or 50 mbar of atmospheric pressure with 20 mbar nitrogen, 20 mbar oxygen, 6 mbar water vapor, and less than 1 mbar of carbon dioxide comprising the atmosphere. A fabric only 0.2-mm thick would be sufficient for a 50-meter dome if it were used as a greenhouse only. Such a dome, enclosing about 2,000 square meters (a half acre) of crop land, would have a fabric mass of about one tonne, but its Plexiglas shield mass would still be 4 tonnes. (The Plexiglas geodesic dome shield mass could be cut almost in half if the upper hemisphere of the dome is shaped as a lens, instead of a conventional half sphere. Lens shaping of the upper hemisphere would also make construction of the shield dome easier, as it would not have to go as high. It would also drastically cut the time the plants would need to oxygenate the dome’s atmosphere.) However, while plants can tolerate 0.7 psi, humans can’t, and using such low-pressure domes would force those inside to wear spacesuits. Raising the dome pressure to 2.5 psi would eliminate the need for spacesuits. However, unless the base was suffering from a severe shortage of farm land, it probably makes the most sense to make the greenhouse domes suitable for operation at the same 5 psi pressure as the habitation domes. That way tunnels can be built allowing humans in shirtsleeves to pass freely between the two types of domes without having to undergo compression/decompression operations. Moreover, common construction elements would make mass production easier and also allow humans to move into the greenhouse domes as population pressures require. The main difference between the two types of domes will be in the carbon dioxide partial pressures allowed. In the habitation domes, this will be limited to typical terrestrial levels of about 0.4 mbar. But in the greenhouses, much larger carbon dioxide levels of about 7 mbar (Mars ambient) will be employed, as this should greatly i
ncrease crop yields. (Plants on Earth suffer from carbon dioxide deprivation.) As we have seen, there are a variety of potential techniques for supplying the greenhouse with copious supplies of water. Thus, the basic prerequisites for farming—well lit, irrigated land—can be brought into being on Mars.
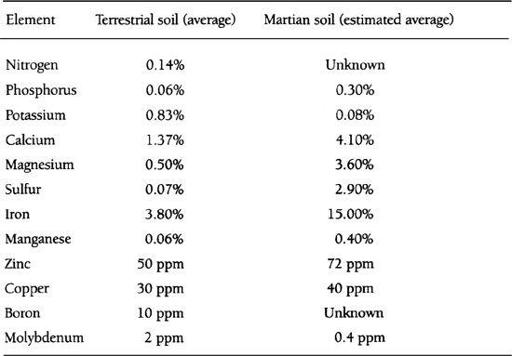
How fertile is Martian land? It’s hard to say, but on the basis of what we know now, Martian soil is likely to prove an excellent medium for crop growth, considerably better than most land on Earth, in fact. In
Table 7.1
we show a comparison of plant nutrient elements in terrestrial and Martian soils. The data for Martian soils is based upon
Viking
results and analysis of SNC meteorites.
25
TABLE 7.1
Comparison of plant Nutrients in Soils on Earth and Mars