The Cerebellum: Brain for an Implicit Self (23 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

Memory sites.
Studies of the VOR have emphasized an important problem concerning its adaptation mechanisms. Several groups have considered whether the memory of VOR adaptation is stored in the cerebellar cortex, vestibular nuclei, or both (
Miles and Lisberger, 1981
;
Lisberger and Sejnowski, 1993
;
Paster et al. 1994
;
Raymond et al., 1996
;
Ito, 1998
;
Broussard and Kassardjian, 2004
). In evaluating this issue, it is well to remember that two phases of VOR adaptation, fast and slow, have been distinguished. In studies on goldfish (
McElligott et al., 1998
) and monkeys (
Nagao and Kitazawa, 2003
), the short-term (fast) VOR adaptation, which developed during about one hour of continuous rotation, was abolished by inducing localized anesthesia of the flocculus. In contrast, slow VOR adaptation was established by repeated trials of VOR adaptation for several days (
van Alphen and De Zeeuw, 2002
). The slow VOR adaption persisted after the flocculus was injected with either an AMPA/kainate receptor blocker, CNQX (cats;
Kassardjian et al., 2005
) or a local anesthetic (monkeys;
Anzai et al., 2010
). Studies on the OKR have also provided important results (
Shutoh et al., 2006
; see later). The implication of these results will be discussed later as common problems for the VOR, OKR, and OFR (
Chapter 12
,
Sections 3-4
).
It has long been known that a unilateral labyrinthectomy causes the development of pronounced nystagmus (
Vidal et al., 1998
). After the lesion, the static deficits generally disappear in a few days, whereas restoration of the dynamic, vestibular-related synergies is much slower and only partial. When the flocculus was lesioned unilaterally 40–70 days earlier, recovery from the effects of the contralateral labyrinthectomy was severely delayed. When such lesions were made after recovery for 16–60 months, they produced only a transient asymmetry of the vestibuloocular responses. These observations suggest that the flocculus is required for initiating
(but not maintaining) the compensatory process following peripheral lesions of the vestibular system (cat;
Courjon et al., 1982
). The behavioral recovery from unilateral labyrinthectomy was accompanied by an asymmetric expression of isoforms of PKC in flocculus Purkinje cells. There was also a regionally selective increase in the number of PKC-immunoreactive Purkinje cells contralateral to the lesion (rat;
Goto et al., 1997
). In another study, the compensation was retarded after application of PKC inhibitors through the cerebral ventricle (rat;
Balaban et al., 1999
). It thus appears that conjunctive LTD, which requires PKC, has a role in vestibular compensation.
Certain changes related to vestibular compensation have been found to occur also in the brainstem. Unilateral labyrinthectomy caused an increase in the number of GABAergic neurons in vestibular nuclei (cat;
Tighilet and Lacour, 2001
), an increase in mRNA coding BDNF in medial vestibular nucleus (mouse;
Li et al., 2001
), and an increase in glycinergic quantal current amplitudes and frequency of glycinergic quantal events in medial vestibular nuclear neurons (mouse;
Lim et al., 2009
). A neuronal network simulation also suggested a change in commissural inhibitory connections after this lesion (
Graham and Dutia, 2001
). Together with cerebellar adaptation by the flocculus, considerable reorganization of the vestibular neuronal network appears to underlie vestibular compensation.
The OKR moves an eye to follow a relatively slowly moving visual environment. When the subject moves in the light, this reflex and the VOR are evoked simultaneously, and they act synergistically. It has been shown that the VOR is especially effective for higher frequencies of a changing visual environment, whereas the OKR is more effective during lower frequencies. For any given frequency, the two systems in combination produce a rather constant gain (~0.8) within the frequency range tested (0.05–2 Hz) (rabbit;
Collewijn and Grootendorst, 1979
). The OKR shares a large part of the neuronal circuit for the VOR. Neurons responding to horizontal optokinetic stimuli have been located in the nucleus reticularis tegmenti pontis (NRTP) (rabbit;
Kano et al., 1991
). In another species, however, these neurons responded to both horizontal optokinetic and vestibular stimulation. During optokinetic stimulation, the response of these neurons was either unidirectional (51%) or bidirectional (49%). All such neurons were shown to exhibit a response during sinusoidal head rotation. Phase and gain analyses suggested that neurons in the (NRTP) convey a head velocity signal (rat;
Taillanter and Lannou, 1988
). Yet to be investigated is whether this nucleus contains a group of neurons specifically devoted to OKR adaptation.
The OKR may appear to be a feedback control mechanism, but in view of the relatively long loop time (~50 ms) consumed for visual information processing, feedback control may be efficient only at a relatively low velocity of optokinetic stimulation. Furthermore, while the OKR has a relatively high open loop gain at a relatively low velocity of the optokinetic stimulus, the open loop gain was shown to decrease steeply at a relatively high velocity (
Collewijn, 1969
). With such a retinal delay and a low open loop gain, the OKR seems unable to perform efficient feedback control by itself at high stimulus velocities. The flocculus appears to intervene in the OKR to cope with these difficulties. The OKR has indeed been shown to exhibit an adaptive gain increase toward unity during prolonged sinusoidal rotation of the visual environment around a stationary subject (rabbit and mouse;
Nagao, 1988
,
1989
;
Katoh et al., 1998
) (
Figure 30
). In another study, OKR adaptation was blocked by the local injection of a NO synthase inhibitor that blocked conjunctive LTD (
Chapter 7
, “
Conjunctive Long-Term Depression (LTD)
”). This adaptation was absent in mutant mice that lacked conjunctive LTD because of their lack of the neuronal isoform of NO synthase (
Katoh et al., 2000
). Also, it was shown that intravenous injection of cyclooxygenase-2 inhibitor (nimesulide), which blocked conjunctive LTD in cerebellar slices, effectively suppressed OKR adaptation (mouse;
Le et al., 2010
). It is also worth noting that LTD induction is facilitated in Delphilin-deficient mutant mice, in which the gain-increase adaptation of the OKR is also enhanced (
Takeuchi et al., 2008
). This may suggest that LTD induction at parallel fiber-Purkinje cell synapses is a crucial rate-limiting step in OKR adaptation.
Figure 30. Adaptation of the optokinetic eye-movement response (OKR).
In an awake mouse, eye movements were recorded via a half mirror using an infrared television camera for real-time recording. A left side, side view of the recording arrangement. IR, infra-red. A right side, top-down view of the animal’s relation to an oscillating screen. “Screen” indicates movement of the screen. B, OKR gain measured before ( ) and after (
) and after ( ) daily training for one hour, repeated for five consecutive days. These mice were kept in the dark during trials. Average values for 12 mice are plotted. Recovery thereafter is followed for two weeks in 4 mice (
) daily training for one hour, repeated for five consecutive days. These mice were kept in the dark during trials. Average values for 12 mice are plotted. Recovery thereafter is followed for two weeks in 4 mice ( ). The OKR gain increase during one hour of screen oscillation implies the occurrence of short-term adaptation (see downward arrows), whereas the increase over five days (thick arrow) indicates long-term OKR adaptation. C shows records of the eye movements of one mouse during screen oscillation, at the first, third, fourth, and sixth day of training. Data for ten or more cycles are averaged. (Courtesy of Soichi Nagao.)
). The OKR gain increase during one hour of screen oscillation implies the occurrence of short-term adaptation (see downward arrows), whereas the increase over five days (thick arrow) indicates long-term OKR adaptation. C shows records of the eye movements of one mouse during screen oscillation, at the first, third, fourth, and sixth day of training. Data for ten or more cycles are averaged. (Courtesy of Soichi Nagao.)
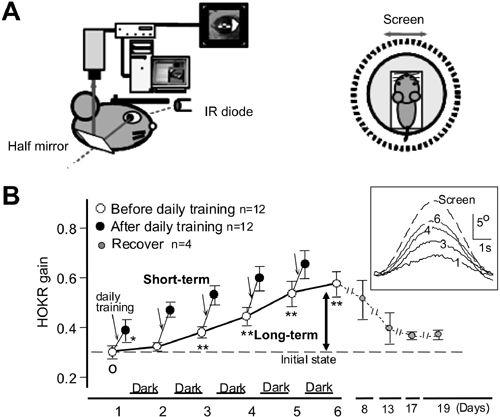
The OKR provides a convenient means of examining cerebellar adaptation because it is so easy to measure (
Figure 30
). Its short-term (fast) adaptation was shown to develop during a 1-hour screen rotation that diminished throughout the subsequent 24 hours. In contrast, long-term (slow) adaptation of the OKR was established by repeated bouts of screen rotation throughout seven days, and it persisted for a week even after the flocculus was injected with locally acting lidocaine. There are two possibilities for the relationship between fast and slow adaptation of the OKR. They may occur independently of each other, or consecutively (i.e., one after the other). It has recently been found that protein synthesis inhibitors injected locally into the flocculus had little effect on fast adaptation, but they blocked slow adaptation (
Okamoto et al., 2011
). This finding suggests that the memory of fast OKR adaptation formed in the flocculus may subsequently induce the memory of slow adaptation in vestibular nuclear neurons. Slow OKR adaptation has been shown to be underlain by LTP in vestibular nerve-VOR relay neuron synapses (mouse;
Shutoh et al., 2006
). A puzzling electron microscope study showed that fast OKR adaptation was accompanied by a significant decrease in the AMPA receptor density of parallel fiber-Purkinje cell synapses in the flocculus, whereas slow OKR adaptation was accompanied by a decrease in the number of parallel fiber-Purkinje cell synapses in the flocculus (mouse;
Nakadate et al., 2004
). These observations suggested that the flocculus maintained a trace of conjunctive LTD in the form of a loss of synapses, yet there was no sign of this trace in OKR gain. Clearly, more information is needed to advance understanding of the neuronal mechanisms that underlie fast and slow OKR adaptation.
The OFR is another eye-movement reflex elicited by brief, unexpected movements of a visual scene (monkey;
Kawano and Miles, 1986
;
Miles and Kawano, 1986
). It may involve but is distinct from the OKR. This was revealed in a study wherein bilateral removal of the cerebral occipital lobes depressed the OFR to a residual response that resembled the OKR (monkey;
Zee et al., 1987
). OFRs involve Purkinje cells in the ventral paraflocculus (
Shidara et al., 1993
;
Gomi et al., 1998
). Unlike the OKR, however, the OFR has a visual perception mechanism located in the cerebral association cortex. Cells in this area were shown to discharge during brief, sudden movements of a large-field visual stimulus that elicited an OFR. These cells were located mainly in the medial superior temporal area (MST) and also partly in the middle temporal area (MT). The response properties of MST neurons during ocular following were similar to those of dorsolateral pontine nucleus neurons. This suggested that MST neurons relayed visual information to the ventral paraflocculus via dorsolateral pontine nucleus neurons (
Kawano et al., 1994
). The OFR is an example of a control system in which an elaborate cerebral cortical mechanism is incorporated as an accessory into the primary controller, which is located in the brainstem.
