The Crash Course: The Unsustainable Future of Our Economy, Energy, and Environment (29 page)
Read The Crash Course: The Unsustainable Future of Our Economy, Energy, and Environment Online
Authors: Chris Martenson
Tags: #General, #Economic Conditions, #Business & Economics, #Economics, #Development, #Forecasting, #Sustainable Development, #Economic Development, #Economic Forecasting - United States, #United States, #Sustainable Development - United States, #Economic Forecasting, #United States - Economic Conditions - 2009

- Time
: Decades. Achieving even modest percentage footholds in our macro energy-use profiles will require a colossal investment. But it needs doing and should be done with all possible haste. - Scale
: Absolutely massive. Alternative energy technologies relying on wind, waves, or sun have extremely low (read: unfavorable) “energy densities,” meaning that instead of installing a single power plant, thousands of individual units have to be installed over a much larger area. To simply construct the factories needed to build wind, solar, and other equipment will be a significant undertaking. Serious questions remain as to whether sufficient rare resources exist to build all the panels and wind mills using current technologies. For example, neodymium magnets may run short due to a lack of the neodymium itself, as it is one of the rare-earth elements that China crimped off from the export markets in 2010. - Cost
: At this point, electricity from solar and wind sources isn’t cost-competitive with fossil fuel sources.
32
While estimating the trillions of dollars necessary to make alternatives a viable replacement for petroleum is a difficult prospect, we can easily state that alternatives would be the highest cost of any of the options. But still, these investments should be made.
Natural Gas
Of all of the potential alternative fuels, natural gas is best suited to become a “bridge fuel” that we can use to transition into a new future of less energy. Recent advances in shale bed drilling seem to have opened up vast new supplies of natural gas, although environmental concerns (around the chemicals used to “frac” the tight shale open so the gas will flow, and their effects on water tables) and the issue of rapid depletion of the wells remain to be clarified before these new supplies can be relied upon.
But if the reserve numbers are to be believed, then there is ample supply of natural gas to “fund” a transition period. Of course, we’d have to tap that account wisely and preferentially use whatever there is to build a more resilient and efficient energy infrastructure, not waste it trying to increase retail sales and other forms of consumption. The EROEI is very high for gas wells, believed to be somewhere over 30.
33
However, if we’re seriously and credibly going to use natural gas, then we have to immediately begin the enormous task of retrofitting our energy and transportation infrastructure to use it. Cars will have to be modified, new natural gas fuel tanks must be installed, service stations will need new refueling equipment and storage tanks, pipelines will have to be built, and so on. However, converting a vehicle to run on natural gas is a snap compared to conversion to electricity, and there’s no compelling reason why such conversions should not be done as quickly and as urgently as possible.
As before, there are issues of time, scale, and cost to be considered if we want to credibly exploit natural gas as a meaningful replacement for oil. It’s certainly possible that we can make the switch, but here in 2010, there is no sign that any such plans are even being considered, let alone approaching a scale of implementation that matches the urgency of the situation.
Yes, we could move toward natural gas as a prime energy source. But to do so, we would have to make the shift within a single decade, and no major energy transition has ever been accomplished in that short of a period of time. Is this possible? Sure, anything is possible. Is it probable? Not if we leave it up to “the markets,” which seem to remain blissfully unaware of Peak Oil even as we have already passed the peak of conventional oil and appear ready to pass a peak of all types of oil in just a few years. I won’t get excited by the prospect of a transition to natural gas until I hear the U.S. president get on television and announce the equivalent of a World War II–era effort to immediately begin building out the necessary infrastructure to make it happen.
If there was one area where we might want to pressure our elected officials to support one energy transition over another, it would be for natural gas over corn-based ethanol. Hands down, natural gas is the winner due to its massively higher EROEI. Unless we get serious about making this transition, and soon, there’s not much hope that natural gas will ever do more than play “catch up” with a receding oil horizon.
Conclusion
Together, nuclear, coal, and the alternatives will definitely play a role in our energy future. But none will be the energy savior that some are hoping for (or even counting on). Perhaps if we had started transitioning to these alternatives 10 or even 20 years ago, they could have slipped more comfortably into a lead role, but just like oil, none of them could have provided exponentially more energy forever. That is just the basic reality of living on a finite planet with finite resources.
Unfortunately we did not even begin
mentally
transitioning away from oil in the decades before its imminent peak, let alone structurally or economically. In order to have facilitated any kind of soft landing, several decades of preparation would have been required, given the realities of time, scale, and cost involved.
A set of structural, wrenching, and possibly disruptive changes are on the way. At this point, I’m confident that we will rely more heavily on nuclear, coal, and alternative energy sources in the future because we will have to, but I’m also just as confident that these resources will never be able to fully plug the gap left by depleting oil. We may
hope
that they might, but we shouldn’t count on them. The numbers are too large; the math doesn’t work.
The most probable outcome, given the level of funding priority and other actions by various world leaders, is for these alternative sources to play limited, albeit important, roles in our energy future. Nuclear, coal, and the alternatives can help to mitigate the impacts, but they cannot prevent them.
The implications of this are profound. The economy that you and I have come to know and love—the one predicated on a constant flow of ever-increasing quantities of energy—will have to operate on less energy. Even though having a few percent less energy instead of a few percent more sounds relatively minor, for an intertwined set of economic, financial, and monetary systems that are all based on perpetual exponential growth, the potential impacts are enormous.
CHAPTER 18
Why Technology Can’t Fix This
By now, some of you are probably thinking I’ve seriously underestimated the role that technology and innovation will play in our future. Perhaps I have, but my background as a scientist keeps intruding into my optimism about the ability of technology to solve the predicaments we face. In truth, I love technology and what it has brought us over the past centuries and will bring us in the future. I will stand up and applaud new discoveries and new advances louder than anyone in the crowd—when they are rolled out. But we need to face a few facts and assure ourselves that we haven’t placed too much optimism where it doesn’t belong.
Fact 1—Technology Does Not Create Energy
Because technology seems to produce so many miracles, it’s sometimes easy to forget what it can and cannot do. Technology can help us do things more efficiently and effectively than in the past, and it can help us do far more with less. It can entertain and connect us in ways that we couldn’t have conceived of only a decade ago. It can boost productivity. It can help us transform and use energy through innovative applications. It allows us to connect instantly with each other in new and exciting ways. But it cannot
create
energy.
Energy can neither be created nor destroyed. So says the First Law of Thermodynamics. Energy can only be transformed from one form to another, such as when coal is turned into electricity, which becomes the cold air blown into a dentist’s office in summer. Not once, not ever, not in any laboratory in the world, not even for a millisecond, has technology created more energy than it has used. Energy has certainly been transformed in quite brilliant ways, but the final accounting is always the same: Just as much energy exists as before the transformation; it’s just that some of it is now in the form of diffuse heat that is useless for performing any work.
This is where the Second Law of Thermodynamics comes in. It governs what happens to energy when it’s transformed: Every transformation always loses at least a little energy (and sometimes quite a lot) in the form of diffuse heat. Diffuse heat is the tax that the universe places on all energy transactions. There’s nothing wrong with diffuse heat—those of us in the northern United States happen to love it in our offices in February—it’s just that diffuse heat cannot perform any work, and it’s the work that energy performs that we’re mainly after. It bears repeating:
Every single time we convert energy from one form to another, we lose some of that initial energy content to the universe in the form of heat
.
For example, we might burn coal to turn into electricity, which we then use to split water so that we can capture and use hydrogen. Following this same set of transformations using the Second Law of Thermodynamics as our guide, we get the following: (1) When that coal is burned, about 40 percent of the energy it initially contained goes toward turning the electrical turbines, but 60 percent of its energy is lost to the universe as waste heat. (2) The electricity travels to the site where the water will be split, losing 7 percent of its energy along the way in the form of nicely warmed transmission lines that gently radiate their heat into the universe. (3) The electrolysis is performed, splitting water into oxygen and hydrogen, with 80 percent of the energy in the electricity captured in the form of pure hydrogen and a final 20 percent lost as heat. At every step, the universe demanded and received its tax in the form of diffuse heat. In this example, the final efficiency of converting coal ⇒ electricity ⇒ hydrogen is 0.40 × 0.93 × 0.80 = 30 percent. In other words, the act of converting coal to hydrogen loses 70 percent of the energy in coal to the universe.
The universe always tends toward randomness as it ceaselessly strives toward its goal of someday reaching one very average and uniform temperature. This is the law of entropy. Entropy represents the amount of energy in a system that is no longer available for doing mechanical work. At each stage of our conversion of coal into hydrogen, entropy (randomness) increases. Perhaps confusingly,
lower
entropy means there’s a
higher level of order
in a system. As entropy increases, so does disorder and randomness. Entropy then, is the name of the tax that the universe places on all energy transformations.
Entropy is the reason that your coffee cup starts hot and gets cold, but never starts warm and gets hotter all on its own. Cold molecules are slower-moving, closer together, and more orderly than heated molecules. They have less entropy than warmer molecules. It is the rule of the universe that high entropy always runs toward low entropy and never the opposite, just as running water always heads toward the sea. All molecules with higher disorder (heat energy) seek to share their wild exuberance with molecules that have less disorder, never the other way around. So your coffee cup starts hot but grows cooler, until it has shared as much of its entropy as it can and reaches room temperature. If entropy ever ran in reverse, you’d be as surprised as if you saw a river flowing uphill or a jumbled pile of books fly up onto a shelf in perfect alphabetical order.
Energy comes in many forms, ranging from highly concentrated to diffuse, from extremely useful to utterly useless for the task of performing work. The least-concentrated form of energy is diffuse heat at the background temperature (whatever that happens to be), and that form lives over on the “completely useless” end of the work spectrum. No machine in the world can perform net work from a single temperature reservoir, no matter how much heat energy it contains; work can only be extracted from heat if there’s a temperature difference between two separated reservoirs. For example, there is an enormous amount of heat energy contained within Lake Superior, but we cannot hook a machine up to it and use that energy to perform any work.
The second law states that as we transform energy, we always start with a concentrated form, like diesel fuel or a stick of wood, and after we’ve transformed it into something else, we’re left with whatever work that energy performed plus heat—random, diffuse heat. Our unavoidable entropy tax.
Think of the Second Law of Thermodynamics as a frictionless slide (meaning you can’t wriggle back up the slope to a higher spot), where at the top of the slide is beautiful, wonderful, concentrated energy, and at the bottom is diffuse heat. At the top of the slide we might put heating oil and 50 degree air molecules, and at the bottom we might put 70 degree air molecules. Once that heating oil descends and is converted into heat along the way, the trip down the slide is over and done. Once that heating oil has taken its trip down the slide, no more work can be performed from the energy that the heating oil once contained.
Suppose we put some natural gas, a marvelously concentrated form of energy, at the top of the slide and then use it to turn an electrical generator. The natural gas, when burned, performs some work by turning the generator, while the rest is turned into heated exhaust molecules. In asking the natural gas to perform this work, we nudge it down the slide, and it races to the bottom, while performing some useful work and paying the entropy tax along the way. Work and heat.
When energy takes a trip down that frictionless slide, it’s a one-way trip. Water never flows uphill and burned hydrocarbons never magically reform themselves out of exhaust fumes. Every form of energy gets only one turn on the slide.
Given this, I like to think of the concentrated energy that we have been given as a one-time free gift of energy perched at the top of a frictionless slide. Our choice is whether we’re going to do something truly useful with that energy when we push it down the slide, or simply turn as much of it as we can into useless heat as fast as possible. Either way, we only get to do it once.
In all of history, we’ve never, not once, violated this law of entropy. If we did, it would be the most spectacular news in scientific (and human) history, and many people, especially me, would scour the findings with great excitement to be sure they were true. But we quite regularly read about people who have claimed to violate this law. Nearly every year, claims surface that a perpetual motion machine that produces more energy than it consumes has been invented. The inventors of these magical devices demonstrate a remarkable ability to secure gullible media interest, and sometimes even deep-pocketed investors, but not one of them in all of history has ever produced a surplus-energy-perpetual-motion machine that actually works as claimed. To do so would mean that a perpetual motion machine would be able to nudge some energy down the frictionless slide
and
harvest enough work along the way that the same amount of energy could be pushed back up the slide as was used. You might as well try and lift yourself into the air. Needless to say, the law has yet to be broken, and a perpetual motion machine has yet to be invented.
If you find the Second Law of Thermodynamics a bit esoteric and want to observe a more direct and observable law of nature that has also never been violated, consider the law of gravity. Not once has anything that has been dropped on Earth ever floated upward instead of accelerating downward. Despite our technological prowess, not once have we ever found a way to defeat gravity here on the surface of the earth with our technology. We can temporarily defeat gravity using the powerful forces found in rockets and magnets, but we’ve never permanently diminished it. Just as the Second Law of Thermodynamics has proven to be an immutable law of nature that is immune from our technological reach, so, too, has gravity. The latest high-tech gizmos may intrigue and impress us, but they are as firmly straitjacketed by the laws of energy and entropy as you are glued to the earth by gravity.
Our first step toward understanding the limits of technology is to fully appreciate that technology can find, produce, and transform energy, but it cannot create it. Once we really understand the fact that technology does not (has not, will not,
cannot
) create energy, we’re in a better position to appreciate its offerings and shortcomings.
Fact 2—Transforming Energy Is Expensive
Once energy has taken the trip all the way to the bottom of the frictionless slide, it’s lost to us as a form of energy that can do useful work. Technology will permit us to take less concentrated forms of energy and make them more concentrated, perhaps even push them back up the slide, but only at an energy cost. By now you know this cost is “diffuse heat.” Any time we decide to concentrate a form of energy, we lose some energy to heat. Put another way, if we want to create one unit of concentrated energy, we will have to start with
more than one unit
of less-concentrated (but still useful) energy, with the extra balance representing the portion that will be “donated” to the universe as heat. Pushing things back up the slide is possible, but only if we’re willing to pay.
This is why the much-advertised “hydrogen economy” is an energy bust waiting to happen. There are no existing deposits of hydrogen to mine or tap. Hydrogen is energy-expensive to make, and there’s simply no way to make it without losing energy along the way. We might make it from natural gas, or from electricity, but we lose heat with every step of the conversion process. The more hydrogen we make, the less energy we have. Hydrogen might make sense economically and/or politically, but it’s an energetic flop. I’m picking on hydrogen a bit here, but the principle applies to any and every energy transformation.
And there’s nothing that technology can do to circumvent this reality. Transforming energy is expensive; it costs energy. Heat is lost, the entropy tax is unavoidable. This isn’t techno-pessimism—it’s the law: the Second Law of Thermodynamics, to be exact. The universe always applies its tax; entropy constantly increases.
Fact 3—Energy Transitions Take Time
If Peak Oil arrives before 2020, as seems likely at the time of this writing, then very little time remains to effect a transition to alternative sources of energy, whatever they may be. Energy transitions take time. A lot of time. And that is not so much a function of technology, but of human behavior and the economics of already-deployed capital.
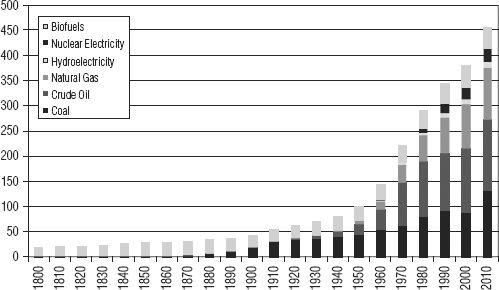
For example, note in
Figure 18.1
how long it took for coal to equal the energy contribution of wood (“biofuels” in the chart), and for oil to then equal the energy from coal, and that natural gas has still not equaled either of those (although it could). Nuclear and hydro remain distant competitors in the energy game.