In Search of Memory: The Emergence of a New Science of Mind (21 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology

On this and on many subsequent occasions, Denise succeeded in turning my attention from what could easily have become—and occasionally was—a full-time preoccupation with science to more immediate involvements with our children. Both for Paul and for our daughter, Minouche, born in 1965, I was a concerned and involved parent but hardly an ideal one. I missed at least half of Paul’s Little League baseball games, including one game in which he stepped up to bat with the bases loaded and hit a bases-clearing double. This feat was heard around the world in our house, and to this day I regret having missed it.
As I was approaching my seventy-fifth birthday in 2004, we celebrated it three months early so we could be joined at our summer home on Cape Cod by our children, their spouses, and our four grandchildren: Minouche and her husband, Rick Sheinfield, and their children, Izzy, five, and Maya, three; and Paul and his wife, Emily, and their two daughters, Allison, twelve, and Libby, eight. Minouche, who graduated from Yale and from Harvard Law, practices public interest law in San Francisco, focusing on women’s issues and women’s rights. Rick is a lawyer for the city and specializes in hospital and health care issues. Paul studied economics at Haverford as an undergraduate and then went on to the Columbia Business School. He manages a set of funds for Dreyfus. Emily graduated from Bryn Mawr and the Parsons School of Design and runs her own interior design firm.
At my birthday dinner, I raised a toast to our children, their spouses, and my four grandchildren. I said that I was proud to see what principled and interesting people our children had turned out to be and how thoughtful they are as parents of their own children, given the fact that I was only a B+ father. Minouche, who enjoys tweaking me, yelled out, “Grade inflation!”
Minouche put my parenting into perspective on another occasion. “You were great, Pops, in giving me the sense that I could do anything intellectually. You read to me often when I was little and you always took a deep interest in what I thought and in my work at Horace Mann, in college, in law school, and even now. But not once, as I remember my childhood, did you ever take me on routine visits to the doctor!”
It was, and still is, understandably difficult for my children to understand—much less excuse—that I find doing science endlessly fascinating and that my involvement in it can expand almost infinitely. It has required conscious effort on my part and help from Denise and from my psychoanalysis to be more realistic and to structure my time so as to make room for the responsibilities and pleasures of my life with Minouche and Paul and with their children.
SPENDING MORE TIME AT HOME WITH DENISE AND PAUL ALSO
gave me more time to think about how to approach the study of learning in
Aplysia
. Alden Spencer and I had found few differences in the basic properties of neurons that participate in memory storage and those that do not. Those findings supported the idea that memory relies not on the properties of the nerve cell per se but on the nature of the connections between neurons and how they process the sensory information they receive. This led me to think that in a circuit that mediates behavior, memory may result from changes in synaptic strength brought about by certain patterns of sensory stimulation.
The basic idea that some type of change in synapses might be important for learning had been proposed by Cajal in 1894:
Mental exercise facilitates a greater development of the protoplasmic apparatus and of the nervous collaterals in the part of the brain in use. In this way, pre-existing connections between groups of cells could be reinforced by multiplication of the terminal branches…. But the pre-existing connections could also be reinforced by the formation of new collaterals and…expansions.
A modern form of this hypothesis was put forward in 1948 by the Polish neuropsychologist Jerzy Kornorski, a student of Pavlov. He argued that a sensory stimulus leads to two types of changes in the nervous system. The first, which he called excitability, follows the generation of one or more action potentials in a neuronal pathway in response to a sensory stimulus. The firing of action potentials briefly raises the threshold for generating additional action potentials in those neurons, a well-known phenomenon called the refractory period. The second, more interesting change, which Kornorski called plasticity, or plastic change, leads, he wrote, to “permanent functional transformations…in particular systems of neurons as a result of appropriate stimuli or their combination.”
The idea that certain systems of neurons were highly adaptable and plastic and could therefore be changed permanently—perhaps because of a change in the strength of their synapses—was now very appealing to me. This raised the question in my mind: How do these changes come about? John Eccles had been very enthusiastic about the possibility that synapses change in response to excessive use, but when he tested the idea, he found that they changed for only a brief period of time. “Unfortunately,” he wrote, “it has not been possible to demonstrate experimentally that excess use produces prolonged changes in synaptic efficacy.” To be relevant for learning, I thought, synapses would have to change for long periods of time—in extreme cases, as long as the lifetime of the animal. It now dawned on me that perhaps Pavlov was so successful in producing learning because the simple patterns of sensory stimulation he used elicited certain natural patterns of activation that were particularly suited for producing long-term changes in synaptic transmission. This idea really caught my fancy. But how to test it? How was I going to elicit this optimal pattern of activity?
With further reflection I decided to try to simulate in the nerve cells of
Aplysia
the patterns of sensory stimulation that Pavlov had used in his learning experiments. Even if initiated by artificial means, such patterns of activity might reveal some of the long-term plastic changes of which synapses are capable.
AS I BEGAN TO THINK SERIOUSLY ABOUT THESE IDEAS, I REALIZED
that I would need to reformulate Cajal’s theory that learning modifies the strength of the synaptic connections between neurons. Cajal thought of learning as a single process. Because I was familiar with the behaviorist work of Pavlov and the later cognitive psychological studies of Brenda Milner, I realized that there are many different forms of learning produced by different patterns and combinations of stimuli and that these give rise to two very different forms of memory storage.
I therefore extended Cajal’s idea in the following manner. I presumed that different forms of learning give rise to different patterns of neural activity and that each of these patterns of activity changes the strength of synaptic connections in a particular way. When such changes persist, the result is memory storage.
Restating Cajal’s theory in these terms enabled me to consider ways of translating Pavlov’s behavioral protocols into biological protocols. After all, habituation, sensitization, and classical conditioning—the three learning protocols described by Pavlov—are essentially a series of instructions on how a sensory stimulus should be presented, alone or in combination with another sensory stimulus, to produce learning. My biological studies would be designed to determine whether different patterns of stimuli, modeled on Pavlov’s forms of learning, would give rise to different forms of synaptic plasticity.
In habituation, for example, an animal that is repeatedly presented with a weak or neutral sensory stimulus learns to recognize the stimulus as unimportant and ignores it. When a stimulus is strong, as in sensitization, the animal recognizes the stimulus as dangerous and learns to enhance its defensive reflexes in preparation for withdrawal and escape; even an innocuous stimulus presented shortly thereafter will elicit an enhanced defensive response. When a neutral stimulus is paired with a potentially dangerous stimulus, as in classical conditioning, the animal learns to respond to the neutral stimulus as if it were a danger signal.
I thought that I should be able to elicit, in the neural pathways of
Aplysia
, patterns of activity similar to those evoked in animals undergoing training in these three learning tasks. I could then determine how synaptic connections are changed by patterns of stimuli that simulate different forms of learning. I called this approach neural analogs of learning.
I was guided to this idea by an experiment that was reported just as I was contemplating how to begin my experiments on
Aplysia
. In 1961, Robert Doty at the University of Michigan in Ann Arbor made a remarkable discovery about classical conditioning. He applied a weak electrical stimulus to a part of the dog’s brain governing vision and found that it produced electrical activity in neurons of the visual cortex, but no movement. Another electrical stimulus applied to the motor cortex caused the dog’s paw to move. After a number of trials in which the stimuli were paired, the weak stimulus alone elicited movement of the paw. Doty had clearly shown that classical conditioning in the brain does not require motivation: it simply requires the pairing of two stimuli.
This was a big step toward a reductionist approach to learning, but the neural analogs of learning that I wanted to develop required two further steps. First, instead of conducting experiments in whole animals, I would remove the nervous system and work on a single ganglion, a single cluster of about two thousand nerve cells. Second, I would select a single nerve cell—a target cell—in that ganglion to serve as a model of any synaptic changes that might occur as a result of learning. I would then apply different patterns of electrical pulses modeled on the different forms of learning to a particular bundle of axons extending from sensory neurons on
Aplysia
’s body surface to the target cell.
To simulate habituation, I would apply repeated, weak electrical pulses to this neural pathway. To simulate sensitization, I would stimulate a second neural pathway very strongly, one or more times, and see how it affected the target cell’s response to weak stimulation of the first pathway. Finally, to simulate classical conditioning, I would pair the strong stimulus to the second pathway with the weak stimulus to the first pathway in such a way that the strong stimulus would always follow and be associated with the weak stimulus. In this way, I could determine whether the three patterns of stimulation altered the synaptic connections with the target cell and, if so, how. Different changes in synaptic strength in response to the three different patterns of electrical stimulation would represent analogs—biological models—of the synaptic changes in
Aplysia’s
nervous system brought about by training for the three different forms of learning.
I wanted these neural analogs to answer one key question: How are synapses changed by different patterns of carefully controlled electrical stimuli that mimic the sensory stimuli in the three major learning experiments? How, for example, are synapses modified when, as in classical conditioning, a weak stimulus to one pathway immediately precedes, and therefore predicts, a strong stimulus to another pathway?
To answer this question, I applied in January 1962 for an NIH postdoctoral fellowship that would enable me to work in Tauc’s laboratory. My specific aim was
to study the cellular mechanisms of electrophysiological conditioning and of synaptic usage in a simple nerve network…. This exploratory study will attempt to develop methods of conditioning a simple preparation and of analyzing some of the neural elements in this process…. The long-range goal is to “trap” a conditioned response in the smallest possible neural population in order to permit a multiple microelectrode investigation of the activity of the participating cells.
I ended my application with these words:
It is an explicit hypothesis of this research that the potentiality for elementary forms of conditioned plastic change is an inherent and fundamental property of all central nervous collectivity, whether simple or complex.
I was testing the idea that the cellular mechanisms underlying learning and memory are likely to have been conserved through evolution and therefore to be found in simple animals, even when using artificial modes of stimulation.
The German composer Richard Strauss commented that he often wrote his best music after an argument with his wife. This has not generally proven to be the case for me. But the argument with Denise about spending more time with her and Paul did cause me to pause and think. As a consequence I learned from this argument the obvious lesson that hard thinking, especially if it leads to even one useful idea, is much more valuable than simply running more experiments. I was later reminded of a comment made about Jim Watson by Max Perutz, the Viennese-born British structural biologist: “Jim never made the mistake of confusing hard work with hard thinking.”
In September 1962, with an NIH stipend assuring us a grand annual salary of $10,000, Denise, Paul, and I set off for a fourteen-month stint in Paris.
The century that is ending has been preoccupied with nucleic acids and proteins. The next one will concentrate on memory and desire. Will it be able to answer the questions they pose?
—François Jacob,
Of Flies, Mice and Men
(1998)
B
eing in Paris was wonderful, and I grew accustomed to spending time walking around the city every weekend with Denise and with Paul, which made the experience of being in France rewarding for all of us. In addition, I was pleased to be doing science full-time again. Ladislav Tauc and I complemented each other’s interests and areas of competence, making him an excellent person with whom to work. Besides being completely at home with
Aplysia
, Tauc had trained in physics and biophysics, areas that are fundamental to cellular physiology. I lacked a strong background in either area and learned a good deal about them from him.
Born in Czechoslovakia, Tauc (figure 11–1) had obtained his Ph.D. by studying the electrical properties of large plant cells, which have a resting potential and an action potential similar to those of nerve cells. He brought this interest to bear on
Aplysia
, studying the largest cell in the abdominal ganglion, a cell I later called R2, and describing the site within this neuron where the action potential is generated. Since his focus was on the biophysical properties of nerve cells, he had not studied neuronal circuits or animal behavior and had given little thought to learning and memory, the issues that dominated my thinking about the mammalian brain.

11–1
Ladislav Tauc (1925–1999) was a pioneer in the study of
Aplysia
. I worked with him for a fourteen-month period in Paris and in Arcachon, France in 1962–63. (Reprinted from
Cellular Basis of Behavior
, E. R. Kandel, W. H. Freeman and Company, 1976.
Like many good postdoctoral experiences, mine did more than simply enable me to benefit from a senior scientist’s considerable background and experience. It also allowed me to contribute intellectually by bringing my own knowledge and experience to bear on our common work. Originally, Tauc was a bit skeptical about trying to study learning on the cellular level in
Aplysia
. But in time, he became enthusiastic about my plan of studying analogs of learning in single cells in the abdominal ganglion.
As I had planned when thinking about this research, I dissected out the abdominal ganglion, with its two thousand nerve cells, and mounted it in a small chamber bathed with aerated seawater. I placed microelectrodes inside one cell, usually cell R2, and then recorded that cell’s responses to various sequences of stimuli applied to the neural pathways that converged on it. I used three patterns of stimulation, based on Pavlov’s work in dogs, to develop three analogs of learning: habituation, sensitization, and classical conditioning. In classical conditioning, an animal learns to respond to a neutral stimulus in the same way it would respond to an effective, threatening or negative stimulus. That is, it forms an association between the neutral stimulus and the negative stimulus. In habituation and sensitization, an animal learns to respond to one type of stimulus without associating it with any other stimulus. The experiments proved even more effective than I had anticipated.
Through habituation, the simplest form of learning, an animal learns to recognize a stimulus that is harmless. When an animal perceives a sudden noise, it initially responds with several defensive changes in its autonomic nervous system, including dilation of the pupils and increased heart and respiratory rates (figure 11–2). If the noise is repeated several times, the animal learns that the stimulus can safely be ignored. The animal’s pupils no longer dilate and its heart rate no longer increases when the stimulus is presented. If the stimulus is removed for a period of time and then presented again, the animal will respond to it again.
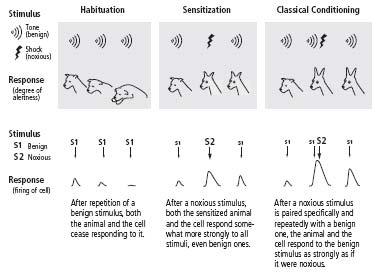
11–2 Three types of implicit learning
. Habituation, sensitization and classical conditioning can be studied both in animals (above) and in individual nerve cells (below).
Habituation enables people to work effectively in an otherwise noisy environment. We become accustomed to the sound of the clock in the study and to our own heartbeat, stomach movements, and other bodily sensations. These sensations then enter our awareness rarely and only under special circumstances. In this sense, habituation is learning to recognize recurrent stimuli that can safely be ignored.
Habituation also eliminates inappropriate or exaggerated defensive responses. This is illustrated in the following fable (with apologies to Aesop):
A fox that had never yet seen a turtle was so frightened when he encountered one in the forest for the first time that he nearly died. On his meeting with the turtle for the second time, he was still much alarmed but not to the same extent as at first. On seeing the turtle for the third time, he was so increased in boldness that he went up to him and commenced a familiar conversation with him.
The elimination of responses that fail to serve a useful purpose focuses an animal’s behavior. Immature animals often show escape responses to a variety of nonthreatening stimuli. Once they become habituated to such stimuli, they can focus on stimuli that are novel or associated with pleasure or danger. Habituation is therefore important in organizing perception.
Habituation is not restricted to escape responses: the frequency of sexual responses can also be decreased through habituation. Given free access to a receptive female, a male rat will copulate six or seven times over a period of one or two hours; afterward, he appears sexually exhausted and becomes inactive for thirty minutes or longer. This is sexual habituation, not fatigue. An apparently exhausted male will promptly resume mating if a new female is made available.
Because of its simplicity as a test for recognizing familiar objects, habituation is one of the most effective means of studying the development of visual perception and memory in infants. Infants characteristically respond to a novel image with dilated pupils and increased heart and respiratory rates. If shown an image repeatedly, however, they will stop responding to it. Thus an infant who has been repeatedly shown a circle will ignore it. But if the infant is then shown a square, its pupils will again dilate, and its heart and respiratory rates will increase, indicating that it can distinguish between the two images.
I modeled habituation by applying a weak electrical stimulus to a bundle of axons leading to cell R2 and then repeating that stimulus ten times. I found that the synaptic potential produced by the cell in response to the stimulus decreased progressively with repetition. By the tenth stimulus, the response was only about one-twentieth as strong as it had been initially, just as an animal’s behavioral response abates when a neutral stimulus is presented repeatedly (figure 11–2). I called this process homosynaptic depression: depression because the synaptic response was decreased, and homosynaptic because the depression occurred in the same neural pathway that was stimulated (
homo
means “the same” in Greek). After withholding the stimulus for ten or fifteen minutes, I applied it again and saw that the cell’s response returned to almost its initial strength. I called this process recovery from homosynaptic depression.
Sensitization is the mirror image of habituation. Instead of teaching an animal to ignore a stimulus, sensitization is a form of learned fear: it teaches the animal to attend and respond more vigorously to almost any stimulus after having been subjected to a threatening stimulus. Thus, immediately after a shock has been delivered to an animal’s foot, for instance, the animal will exhibit exaggerated withdrawal and escape responses to a bell, a tone, or a soft touch.
Like habituation, sensitization is common in people. After hearing a gun go off, a person will show an exaggerated response and will jump when he hears a tone or senses a touch on the shoulder. Konrad Lorenz elaborates on the survival value of this learned form of arousal for even simple animals: “An earthworm that has just avoided being eaten by a blackbird…is indeed well advised to respond with a considerably lowered threshold to similar stimuli because it is almost certain that the bird will still be nearby for the next few seconds.”
In modeling sensitization I applied a weak stimulus to the same neural pathway leading to cell R2 that I had used in my earlier experiments on habituation. I stimulated it once or twice to elicit a synaptic potential that would serve as a baseline measure of the cell’s responsiveness. I then applied a series of five stronger stimuli (designed to simulate disagreeable or noxious stimuli) to a different pathway leading to cell R2. After I had presented the stronger stimuli, the cell’s synaptic response to stimulation of the first pathway was greatly enhanced, indicating that synaptic connections in that pathway had been strengthened. The enhanced response lasted up to thirty minutes. I called this process heterosynaptic facilitation: facilitation because synaptic strength was enhanced, and heterosynaptic because the enhanced response to stimulation of axons in the first pathway was brought about by strongly stimulating a different pathway (
hetero
means “different” in Greek) (figure 11–2). The enhanced response to the first pathway depended only on the greater strength of the stimulus to the different pathway and not on any pairing of weak and strong stimuli. Thus it resembled behavioral sensitization, a nonassociative form of learning.
Finally, I tried to simulate aversive classical conditioning. This form of classical conditioning teaches an animal to associate an unpleasant stimulus, such as an electric shock, with a stimulus that ordinarily elicits no response. The neutral stimulus must always precede the aversive stimulus and in this way will come to predict it. For example, Pavlov used a shock to a dog’s paw as an aversive stimulus. The shock caused the animal to raise and withdraw its leg, a fear response. Pavlov found that after several trials in which he paired the shock with the ringing of a bell—first sounding the bell and then administering the shock—the dog would withdraw its leg whenever the bell sounded, even if no shock followed. Thus, aversive classical conditioning is an associative form of learned fear (figure 11–2).
Aversive classical conditioning resembles sensitization in that activity in one sensory pathway enhances activity in another, but it differs in two ways. First, in classical conditioning an association is formed between a pair of stimuli that occur in rapid sequence. Second, classical conditioning enhances an animal’s defensive responses to the neutral stimulus only, not to environmental stimuli in general, as sensitization does.
Therefore, in my experiments on aversive classical conditioning in
Aplysia
, I repeatedly paired a weak stimulus to one neural pathway with a strong stimulus to another pathway. The weak stimulus came first and acted as a warning of the strong stimulus. The pairing of the two stimuli greatly enhanced the cell’s response to the weak stimulus; moreover, that enhanced response was far greater than the cell’s enhanced response to the weak stimulus in the sensitization experiments (figure 11–2). The added boost was critically dependent on the timing of the weak stimulus, which had unfailingly to precede and predict the strong stimulus.
These experiments confirmed what I had suspected—namely, that a pattern of stimulation designed to mimic the patterns used to induce learning in behavioral studies can change the effectiveness of a neuron’s communication with other nerve cells. The experiments clearly showed that synaptic strength is not fixed—it can be altered in different ways by different patterns of activity. Specifically, the neural analogs of sensitization and aversive classical conditioning strengthened a synaptic connection, whereas the analog of habituation weakened the connection.
Thus, Tauc and I had discovered two important principles. First, the strength of the synaptic communication between nerve cells can be changed for many minutes by applying different patterns of stimulation derived from specific training protocols for learned behavior in animals. Second, and even more remarkable, the same synapse can be strengthened or weakened by different patterns of stimulation. These findings encouraged Tauc and me to write in our paper in the
Journal of Physiology
:
