Junk DNA: A Journey Through the Dark Matter of the Genome (20 page)
Read Junk DNA: A Journey Through the Dark Matter of the Genome Online
Authors: Nessa Carey

As the mapping of the chromosomal regions responsible for Prader-Willi syndrome and for Angelman syndrome gathered pace, it became obvious that the two disorders were located in the same region of the genome. At first, the most obvious assumption was that the disorders were caused in genes that were different from each other, but in very close proximity, like two adjacent shops on a street. But eventually it became clear that the disorders were caused by a defect in exactly the same tightly defined region.
Both conditions had the same underlying genetic cause, a loss of a small region on chromosome 15. The parents of the affected children didn’t suffer from either disorder and when researchers analysed their chromosomes, they discovered these were completely intact. The loss of the key region of chromosome 15 happened during formation of eggs or sperm.
c
It was really bizarre that the deletion of small part of a chromosome could cause two conditions that were so different from each other. But the conundrum began to make more sense once researchers demonstrated that it wasn’t just the fact that this small region of chromosome 15 was missing that was important. What mattered was why it was missing. Seventy per cent of children with Prader-Willi syndrome inherited the abnormal chromosome 15 from mutated sperm cells. Seventy per cent of children with Angelman syndrome inherited the abnormal chromosome from mutated egg cells. A little later scientists discovered that 25 per cent of the patients with Prader-Willi syndrome had two perfectly intact chromosomes; nothing was missing. The problem in these patients was that they had inherited both their copies of chromosome 15 from their mother, instead of one from each parent.
d
In a smaller percentage of Angelman syndrome, the patients had two perfect copies of chromosome 15, but both inherited from their father.
These inheritance patterns make sense only in the context of imprinting, as shown in Figure 10.3. In all the abnormal situations, the cells are lacking an imprinting control region from one parent. This results in abnormal expression levels of the genes that should normally be kept under tight parent-of-origin control, and this leads to pathology including under- or over-growth.
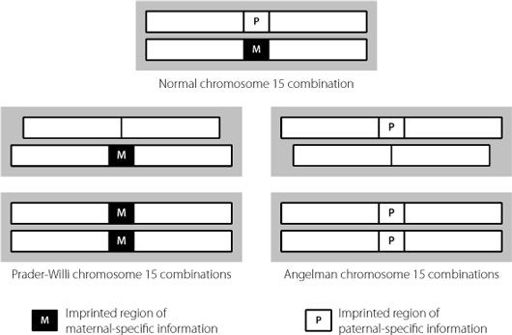
Figure 10.3
Normally, we inherit one copy of chromosome 15 maternally and one paternally. If both copies are inherited maternally, the affected child will have Prader-Willi syndrome. This is also the case if the copy inherited from the father has lost the imprinted region that carries the paternal signature of epigenetic modifications. Essentially, a lack of paternal-specific information leads to Prader-Willi syndrome. Angelman syndrome is caused by a defect in exactly the same region of chromosome 15, but in this case the condition is caused by a lack of maternal-specific information.
Researchers have been able to narrow down the problems that result in these conditions even further, by analysing the genes that are controlled by the imprinting control regions. In about 10 per cent of cases of Angelman syndrome, the patients have inherited all the appropriate DNA from each parent. The problem they have is that there is a mutation in the DNA from their mother. This is not in the ICE but in a gene controlled by the ICE. This is a protein-coding gene, which is normally expressed only from the maternal chromosome. The version on the paternal chromosome is kept silent by imprinting. If the maternally derived gene can’t create
protein because of a mutation, it means the cell can’t produce any of this protein at all, and this leads to pathology.
e
The situation in Prader-Willi syndrome is rather more peculiar. A small number of patients have been identified who are lacking just one of the genes that is found in the critical region on chromosome 15. This gene doesn’t code for a protein. Instead, it codes for a batch of non-coding RNAs, all of which have similar functions.
21
,
22
,
23
These functions are involved in the control of yet another class of RNA molecules that don’t code for proteins. It seems to be the absence of this one non-protein-coding gene that is critical for the majority of symptoms in Prader-Willi syndrome.
Consider the implications. A region of junk DNA (the ICE) controls the expression of a piece of junk DNA that encodes a long non-coding RNA. This long non-coding RNA in turn critically regulates the expression of a gene that codes for a batch of non-coding RNAs. And the role of these non-coding RNAs is to regulate other RNAs that don’t code for proteins. When we think of it in these terms, it becomes quite difficult to argue that junk DNA has no function.
Prader-Willi and Angelman syndromes are not the only human conditions whereby defects in imprinting lead to abnormalities in growth plus associated problems such as learning disabilities. Another reciprocal pair of conditions are Silver-Russell syndrome,
24
an under-growth condition, and Beckwith-Wiedemann syndrome,
25
which is characterised by over-growth. The two conditions are caused in some patients by parent-of-origin issues at the same region of chromosome 11. It’s a particularly complex imprinting locus, with lots of genes involved and more than one ICE.
Similar relationships can be found at other chromosomes.
Children who inherit both copies of chromosome 14 from their mother are growth-restricted in the pre- and post-natal periods but later become obese.
26
But if both copies of chromosome 14 are obtained from the father, an abnormally large placenta develops and the child is born with different problems including defects in the abdominal wall.
27
,
28
For most of these disorders, there are also rare examples of the condition developing because of epigenetic mistakes. There are small numbers of patients who have inherited the correct DNA from the correct parent. The DNA is not mutated and yet the patients develop an imprinting condition. In these rare cases, the establishment and maintenance of the imprint in the zygote and in early development has usually gone wrong. This can result in an ICE being inappropriately methylated or non-methylated and switched off or on when it shouldn’t be. This once again demonstrates the importance of the cross-talk between junk DNA and the epigenetic machinery.
The impact of a dramatic event
In 1978 a little girl called Louise Brown was born. If you had seen Louise Brown you would have thought she was a perfectly ordinary baby. No doubt her parents thought she was the most remarkable baby in the world. What parent doesn’t think this about their child? But on this occasion Mr and Mrs Brown could be forgiven for making this claim because they were right. Louise Brown’s birth was front-page news all around the world, because she was the first test tube baby.
Her mother’s egg had been fertilised by her father’s sperm in a dish in a lab and then replaced into her mother’s womb. This procedure was used because Louise’s mother’s fallopian tubes were blocked and she couldn’t conceive naturally. The successful birth of Louise Brown opened a new era in treatment of human infertility.
It has been estimated that since that first baby over 5 million children have been born using assisted reproductive technologies.
29
There have been claims that assisted reproductive technologies may result in higher levels of imprinting disorders, especially Beckwith-Wiedemann, Silver-Russell and Angelman syndromes. The concerns arise because the embryos are being cultured in the laboratory during the critical period when imprinting gets established. It may seem strange that we don’t know if there really is a problem or not. Surely with 5 million children to analyse it should be quite straightforward to perform the calculations? But the problem is that imprinting disorders are rare, only occurring naturally at rates of one in several or even tens of thousands. When you are analysing events that are so rare, the statistics can be skewed really easily.
Remember Concorde, one of only two supersonic plane models that ever entered commercial service? For decades it was the safest passenger plane in the world, because there had never been a fatal crash. But following the tragic accident at Paris Charles de Gaulle airport in 2000 in which 109 passengers and crew were killed, it became one of the most unsafe planes, statistically speaking. Of course, this was simply because there were relatively few Concorde flights compared with most airliners and the passenger numbers were small (it was a surprisingly bijou plane inside). Therefore, one event could have a major effect on the statistics if these were calculated in a fairly simplistic fashion.
It’s just the same with imprinting disorders. If you would normally expect to see 50 cases for every 5 million children born, how do you interpret it if you detect 55 among the children born via assisted reproductive technologies? Has the medical intervention led to a 10 per cent increase in imprinting disorders, or is this just statistical noise?
f
We also have to bear in mind that infertility
itself may lead to a slight increase in imprinting problems, which is simply unmasked by the assisted reproductive techniques. It’s possible that sperm or eggs from people with low fertility are more likely to carry imprinting defects, but these only become apparent because they are able to have children thanks to medical technology. In the past, they wouldn’t have been able to reproduce so we wouldn’t have seen the effects of the imprinting defect.
30
It’s one of those confusing situations in biology where what we think we see is possibly distorted because of what’s out of sight.
Footnotes
a
The key proteins are called DNMT3A and DNMT3L, the de novo DNA methyltransferases.
b
This protein is called DNMT1 and it is known as a maintenance DNA methyltransferase.
c
This is known as a de novo mutation, meaning newly arising.
d
This is known as uniparental disomy, in this case maternal uniparental disomy.
e
This gene is called UBE3A. It adds a molecule called ubiquitin to other proteins, and this leads to degradation of those proteins.
f
The numbers here are random ones, just chosen to demonstrate the point.
11. Junk with a Mission
It’s quite possible that the most wonderful and compelling aspect of biology is its glorious inconsistency. Biological systems have evolved in magnificently creative ways, usurping and repurposing processes for completely new uses wherever possible. It means that almost every time we think a theme is emerging, we find exceptions. And sometimes it can be very difficult to unravel which is the norm and which the exception.
Let’s take junk DNA and non-protein-coding RNAs. Based on pretty much everything we have seen so far, it would be perfectly reasonable to develop a hypothesis along the following lines:
When junk DNA encodes a non-protein-coding RNA (junk RNA), the function of the RNA is to act as a kind of scaffold, directing the activity of proteins to particular regions of the genome
.
This hypothesis would certainly be consistent with the roles of long non-coding RNAs. They act as the Velcro between epigenetic proteins and DNA or histones. The proteins frequently operate in a complex, and at least one member of the complex is often an enzyme, i.e. a protein that can bring about a chemical reaction. This can be the reaction that adds or removes epigenetic modifications on DNA or histone proteins, or that adds another base to a growing messenger RNA molecule.
In all these situations, the protein is the verb in the molecular sentence. It’s the ‘doing’ or action molecule.
Attractive as this model sounds, it has one unfortunate flaw.
There is a situation where the roles are entirely reversed. In this reversed situation, the proteins are relatively silent, but the junk RNA acts as an enzyme, causing a chemical change to another molecule.
This sounds so peculiar that it is tempting to assume that it’s a one-off quirky exception. But if that’s the case, it’s a really quite remarkable exception because the junk RNA molecules that have this function account for about 80 per cent of the RNA molecules present in a human cell at any given time.
1
We’ve actually known about these peculiar RNA molecules for decades, making it yet more surprising that we have maintained such a protein-centric vision of our genomic landscape.
