Junk DNA: A Journey Through the Dark Matter of the Genome (23 page)
Read Junk DNA: A Journey Through the Dark Matter of the Genome Online
Authors: Nessa Carey

Scientists studying the inflammatory response were among the first to show that DNA sequences can be co-opted to become enhancers when necessary. In this study, the researchers found that once the inflammatory stimulus was removed, the enhancers didn’t revert to being inert. Instead they continued to be enhancers, ready to up-regulate expression of the relevant genes again, if the cells re-encountered the inflammatory stimulus.
4
It’s probably not a coincidence that these enhancers are regulating genes involved in the response to foreign invaders. This memory in terms of gene expression can be very advantageous for fighting off an infection as efficiently and swiftly as possible.
Epigenetics and enhancers – cross-talk in action
One way in which genetic regions can maintain a memory even after a stimulus has gone away is via epigenetics. Epigenetic modifications can make a region easier to switch on again, by keeping the region in a fairly de-repressed state. In human terms, it’s like a
doctor being on call rather than on holiday. In the example above, the researchers demonstrated that certain histone modifications remained at the ‘new’ enhancers after the inflammatory stimulus was removed, keeping them in a state of readiness.
We are generally starting to make a bit more progress at identifying enhancers by looking at epigenetic modifications, which are independent of the underlying DNA sequences. The modifications can be used as functional markers to show how a specific cell type uses a stretch of DNA. Researchers have also shown that these modifications can change in cancer, creating different patterns of gene expression that may contribute to the cellular alterations that lead to cancer.
5
But even if we can find an epigenetic signature that indicates we may be looking at an enhancer, we still have another problem. We don’t know which protein-coding gene is influenced by a putative enhancer. The only way we can establish this is by disrupting an enhancer using genetic manipulations, and then assessing which genes are directly influenced by this change. This is because enhancer function is different from that of the promoter. Enhancers are not orientation-dependent – they act as enhancers no matter which way they are pointing. The other difference is even more dramatic – enhancers can be a very long way from the protein-coding gene whose expression they are influencing.
There are also far more enhancers than we might expect. A recent comprehensive study looked at the patterns of histone modifications in nearly 150 human cell lines. When they assessed these lines for patterns that looked like enhancers, they found nearly 400,000 candidate enhancer regions.
6
This is far more than required if there was a one-to-one relationship between enhancers and protein-coding genes. It’s even too many if we assume that long non-coding RNAs have enhancers.
The enhancers weren’t all found in every cell type. This is consistent with a model where the same stretch of DNA can have
different functions in different cells, depending on how it is epi-genetically modified.
For many years, we had no clear models of how enhancers really work. We now suspect that in many cases they may be critically dependent on another type of junk: the long non-coding RNAs. In fact, specific classes of long non-coding RNAs may be expressed from the enhancers themselves.
7
Many of the long non-coding RNAs we met in Chapter 8 are involved in repressing expression of other genes. But it is now believed that there is also a large class of long non-coding RNAs that enhance gene expression. This was first suggested to be the case for long non-coding RNAs that regulate neighbouring genes. If expression of the long non-coding RNA was increased experimentally, the expression of the neighbouring protein-coding gene also increased. Conversely, if the expression of the long non-coding RNA was knocked down experimentally, the protein-coding gene also showed lower expression.
8
Further evidence came from analysing the timing patterns for specific long non-coding RNAs and the messenger RNAs they were believed to regulate. Researchers treated cells with a stimulus that they knew caused expression of a specific gene. They found that the enhancing long non-coding RNA was switched on before the messenger RNA from the neighbouring protein-coding gene.
9
,
10
This is consistent with a model where the long non-coding RNA located in the enhancer is switched on in response to a stimulus, and then in turn helps to switch on expression of the protein-coding gene.
The long non-coding RNA doesn’t drive this increase on its own. The process is reliant on the presence of a large complex of proteins. The complex is known as Mediator. The long non-coding RNA binds to the Mediator complex, directing its activity to the neighbouring gene. One of the proteins in the Mediator complex is able to deposit epigenetic modifications on the adjacent
protein-coding gene.
b
This helps to recruit the enzyme that creates the messenger RNA copies which are used as the templates for protein production. There is a consistent relationship between the Mediator complex and the long non-coding RNA. Experimentally generated decreases in expression of either the long non-coding RNA or a member of the complex each lead to decreased expression of the neighbouring gene.
11
The importance of a physical interaction between long non-coding RNAs and the Mediator complex has been shown by a human genetic condition. This disorder is called Opitz-Kaveggia syndrome. Children born with this condition have learning disabilities, poor muscle tone and disproportionally large heads.
12
The affected children have inherited a mutation in a single gene. This codes for the protein in the Mediator complex that interacts with long non-coding RNA molecules.
c
The more that scientists analysed the activity of the Mediator complex, the more interested they became. One of the reasons was that the Mediator complex is responsible for the actions of a group of enhancers with special powers. These are the super-enhancers, and they are particularly important in embryonic stem (ES) cells, the pluripotent cells that have the potential to become any cell type in the human body (
see page 105
).
13
The super-enhancers are clusters of enhancers all acting together. They are about ten times the size of normal enhancers. Because of this, proteins can bind to the super-enhancers at very high levels, much higher than are found on normal enhancers. This allows the super-enhancers to really ramp up expression of the gene they are regulating. But it’s not just the numbers of proteins
that bind that interested the researchers. It’s the identities of these proteins.
As we saw in Chapter 8, ES cells don’t stay pluripotent by chance or passively. In order for ES cells to maintain their potential, they regulate their genes very carefully. Even relatively mild perturbations in gene expression can start to push an ES cell down a pathway that converts it into a specialised cell type. One way of visualising this is to think of a Slinky at the top of a tall flight of stairs. Just the slightest nudge to push it over the edge of the top step is enough to send that Slinky on a very long journey. Perhaps an even better analogy might be a Slinky that is held back from falling down the stairs by a small weight on its trailing end. Remove the weight, and off the Slinky will go.
There are a set of proteins that are absolutely vital for maintaining the pluripotency of ES cells. These are known as master regulators, and they are like the small weight on the trailing end of the Slinky. The master regulators are expressed very highly in ES cells, but at much, much lower levels in specialised cells.
The importance of these proteins was unequivocally demonstrated in 2006. Researchers in Japan expressed a combination of four of these master regulators at very high levels in differentiated cells. Astonishingly, this set in motion a chain of molecular events which culminated in the creation of cells that were almost identical in action to ES cells.
14
This is analogous to a Slinky at the bottom of a flight of stairs moving all the way back up to the top step. The cells created by this route have the potential to be converted into any cell type in the body.
d
This remarkable work, and the research that followed on from it, has generated enormous excitement because potentially we can create replacement cells to treat a large number of disorders. These range from blindness to type 1 diabetes, and from Parkinson’s disease to cardiac failure.
Until this new technology was developed, it was extremely difficult to create appropriate cells to treat human conditions. This is because cells from a different individual usually can’t be implanted into another person. The immune system will recognise the donated cells as foreign and kill them, as if they were an invading organism. But, as shown in Figure 12.1, we now have the potential to make cells that are a perfect match for the patient.
The 2006 work has spawned an industry potentially worth billions of dollars, and also resulted in one of the fastest awards of a Nobel Prize in Medicine or Physiology ever, just six years after the original publication.
15
In normal ES cells, some of these master regulator proteins bind at very high densities to the super-enhancers. The super-enhancers themselves are regulating some key genes that maintain the pluripotent state of the cells. The Mediator complex is also present at very high levels in the same locations. Knocking down the expression of a master regulator, or of Mediator, has very similar effects on the expression of these key genes. The expression
levels drop, making the ES cells more likely to start differentiating into specialised cell types.
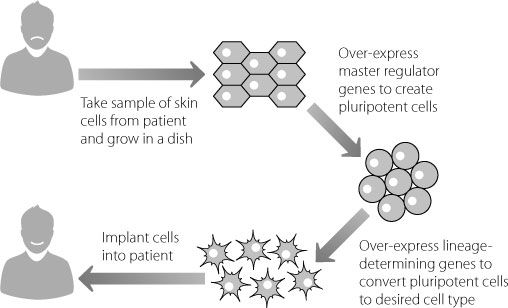
Figure 12.1
The theory behind using patient-derived cells to create therapies tailored for a specific individual.
Because the pluripotent state of ES cells is critically dependent on the expression of high levels of the master regulators, it’s perhaps unsurprising that the master regulators themselves are controlled by super-enhancers. This creates a positive feedback loop, which is shown in Figure 12.2.
Positive feedback loops are relatively rare in biology, mainly because they can be difficult to get back under control if they start to go wrong. Luckily, the protein-coding genes regulated at super-enhancers are extremely sensitive to small perturbations in binding of master regulators and a number of other factors. This may mean that even a small change in the balance of some of these factors may be enough to interrupt this positive feedback loop, and allow the cells to differentiate rather than remain pluripotent. After all, it doesn’t usually take much of a nudge to make a Slinky fall down the stairs.
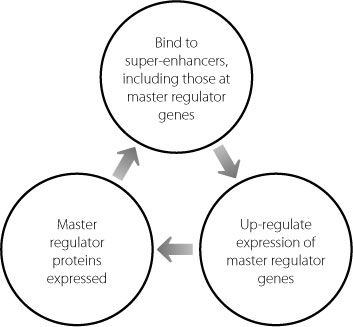
Figure 12.2
The positive feedback loop driving high-level sustained expression of master regulator genes.
Super-enhancers have also been reported in tumour cells, where they are associated with critical genes that drive cell proliferation and cancer progression.
16
One of the genes that is regulated by such a super-enhancer is the same one that we encountered earlier in this chapter, which drives Burkitt’s lymphoma. There are also super-enhancers in some normal specialised cells. These bind cell-specific proteins that define cell identity.
Overcoming the distance
Most of the events described so far involve enhancers that are relatively close to the genes that they target, usually within 50,000 base pairs. It’s relatively easy to visualise how this happens, through the long non-coding RNA and the Mediator complex acting to anchor the enzyme that copies DNA into messenger RNA. But there are a lot of situations where the enhancer and the protein-coding gene that it regulates are a really long distance apart on the chromosome, up to several million base pairs away. This is the difference between trying to pass the salt to someone who is on the other side of the table from you at breakfast, and trying to pass it to someone who is at the other end of a soccer field. It’s quite difficult to visualise how this long-range interaction of gene and enhancer can happen. Neither the long non-coding RNA nor the Mediator complex is large enough to span such a huge distance.
